CellR4 2013; 1 (3): e557
Human Vertebral Body Marrow Processing: Standard Operating Procedure from the cGMP Human Cell Processing Facility of the Cell Transplant Center and Diabetes Research Institute at the University of Miami
Abstract
INTRODUCTION
Vertebral bodies from deceased donors represent a useful source for large scale production of hematopoietic, mesenchymal stem cells (MSCs), as well as subpopulations of human bone marrow (BM)-derived cells for clinical and research applications 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87. Similar protocols have been utilized for generation of BM-derived cellular products in pre-clinical model systems 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97.
At the time of organ retrieval, the vertebral column (VC) is procured from multiorgan donors at the end of other organ procurement and placed in procurement media (PM) 98. Approximately 10-12 VBs are obtained from a VC and processed to extract the bone marrow cells 99. Our extensive experience with VB processing indicates that over 30 billion nucleated donor cells can consistently be obtained from this number of vertebrae. The composition of the PM (manufactured by Mediatech, Inc., Herndon, Virginia) is: DMEM, 2.5% HSA, 50 units/ml Bacitracin, 500 units/ml Polymyxin, 2 mM HEPES and 10 units/ml of Heparin (sodium injection, Elkins Sinn, Inc., Cherry Hill, NJ) are added prior to use 100. The VC is transported to the laboratory. Upon arrival, a sample of the procurement media is taken for microbiological analysis then VC is removed from the procurement media and dipped into Betadine solution. VC is wiped with sterile gauze to remove residual Betadine, followed by rinsing in sterile RPMI 1640 medium. Soft tissue is removed and VC is split through the intervertebral discs into VBs. Cleaned VBs are divided along the sagittal cranio-caudal axis, placed in a sterile beaker and weighed. The vertebral disks are discarded and VBs are cleaned from soft tissues and chopped into smaller pieces. The resulting bone chips are placed into Processing & Re-suspension media (PRM) consisting of RPMI-1640, Calcium/Magnesium and Phenol Red free, with 25 mM Hepes which contains 2.5% HSA, 0.4 mg/ml of Gentamycin, 10 units/ml Heparin (sodium injection, American Pharmaceutical Partners, Inc., Los Angeles, CA) and 2.5 μg/ml DNAse (Dornase alfa, Pulmozyme, Genentech, Inc. San Francisco, CA). The bone chips are processed using an automated oscillating bone grinder (Biorep, Miami, FL) 101 , 102 , 103. The grinder is utilized to crush bone chips, reducing them to fragments. The bone fragments emerging from the machine are placed in PRM (RPMI-1640, Calcium/Magnesium and Phenol Red free, with 25 mM Hepes containing 2.5% HSA, 0.4 mg/ml of Gentamycin, 10 units/ml Heparin, and 0.8 µg/ml of DNAse), and filtered through a 425 and 180 µm sieve assembly. The cells are collected into PRM and set aside at room temperature. Bone fragments recovered from the sieve are placed into a Nalgene jar containing PRM, and the jar is gently shaken (using bone marrow shaker/tumbler) for 30 minutes, to allow for passive release of bone marrow cells from the trabecular framework of the VBs. The resulting suspension is once again filtered through the 425 and 180 µm sieve assembly, followed by centrifugation of cells and collection of retained bone fragments. Bone fragments remaining on the sieve are collected into a Nalgene jar with PRM and are gently shaken for an additional 30 minutes. When this cell suspension is collected through the 425 and 180 µm sieve assembly, all cell suspensions are combined. In order to deplete minute fragments of bone, the cell suspension is centrifuged until the rotor reaches 150xg; at which point the cell suspension and the loose pellet containing the minute bone fragments and chips are separated. The resulting cell suspension is filtered through a 170 µm blood filter (Y-Type Blood Set, McGaw, Inc, Irvine, CA, USA) in order to eliminate cell clumps and bone fragments 104 , 105. An automated cell count using Coulter AcT Diff (Beckman-Coulter Corp., Miami, FL) or a manual cell count (following red blood cell lysis) is performed to obtain a nucleated cell count; cell viability is determined by Trypan Blue exclusion dye. Representative aliquots are collected for microbiological assessment, determination of Endotoxin content, cellular composition and functional capacity. Residual vertebral body trabecular bone chips can be further treated by mechanical and/or enzymatic digestion to extract additional adherent cellular components. The cell products obtained are then ready for further processing, cryopreservation and eventual utilization in selected clinical and research applications.
Human Vertebral Body Marrow Processing Standard Operating Procedures
PURPOSE: To outline the process for separation of human bone marrow cells (BMC) from vertebral bodies (VB) obtained from deceased donors.
RESPONSIBILITY: It is the responsibility of the Operations Director or designee to train cGMP Facility personnel responsible in the execution of this procedure.
SCOPE: This Standard Operating Procedure (SOP) applies to all trained cGMP Facility personnel.
I. GENERAL CONSIDERATIONS
• This procedure must be performed using sterile/aseptic technique 106 , 107. This will involve working within a Biological Safety Cabinet(s) (BSC). Refer to the relevant SOPs for “Sterile/AsepticTechnique” and “Use and Cleaning of Biological Safety Cabinets”.
• Refer to the relevant SOP for “Donor Eligibility Criteria”, which describes the donor acceptance and outlines the necessary testing requirements for allogeneic multiorgan donors, as appropriate for the selected applications.
• Donor testing must be performed by the testing laboratory qualified, licensed and registered by the FDA and acreddited by the CLIA.
• Donors are assessed for
– Infectious disease markers
– ABO group/Rh type
– HLA compatibility
– Red Cell compatibility
Testing results must be documented on relevant SOP, “Request for Processing: Cellular Products of Hematopoietic and Other Origin”.
• VBs can be received from a number of Organ Procurement Organizations (OPO), local or remote.
• If VBs are provided by the local OPO, whose personnel will notify the on-call person when VBs are available for pick up. It is the responsibility of the cGMP personnel to pick up the tissue that become available.
II. DEFINITIONS
A. Vertebral Bodies – The bony segments of the spinal column which are separated by intervertebral disks and cartilage (Figure 1). The origin is human deceased donors.
B. HBM Number – The unique alphanumeric designation for a human bone marrow (the product source is the vertebral column from a deceased donor) product processed in the cGMP Facility. The abbreviation HBM stands for “Human Bone Marrow” followed by 4-5 numbers.
III. PROCESS OUTLINE
The process outline is summarized in Figure 2.
IV. MATERIALS & EQUIPMENT
A. Reagents
NOTE: Only media with the tamper-proof seal present can be used for processing.
NOTE: Processing, Resuspension Media, as well as Transplant/Freezing media will be prepared in-house, using RPMI-1640 (Ca2+/Mg2+, phenol red free) as the base solution.
• Betadine
• RPMI-1640, Ca2+/Mg2+ free, without phenol red, pH 7.4 (Corrning/Mediatech)
• Procurement media (0.9% Sodium Chloride, Normal Saline, injection grade), 500 ml (Corning/Mediatech)
• Hepes, 1 M (Corning/Mediatech)
• 25% Human Serum Albumin, from Bayer or equivalent
• Glutamax, 200 mM (Gibco)
• Gentamycin USP, 40 mg/ml
• Heparin, 1000 U/ml, preservative-free, single use vial, or
• DNAse (Dornase Alfa recombinant Pulmozyme), 1.0 mg/ml, 2.5 ml
B. Equipment
• Biological Safety Cabinets (BSC)
• Table
• Refrigerated centrifuge
• Bone marrow processing machine (Biorep Technologies)
• Bone marrow shaking machine (Biorep Technologies)
• Tube heat sealer
• Sterile connection device
• Balance
• Timer
• Microscope
• AcT Diff Analyzer (Blood Cell Counter)
• Vacuum pump
• Pipette aid
• pH meter
• Refrigerator
C. Instruments & Non Disposables
• Ringstand
• Sterile Human Bone Marrow Isolation pack for the table containing the following items:
1. Sterile large stainless steel tray (1)
2. Sterile medium stainless steel tray (1)
3. Sterile stainless steel bowls (2)
4. Instrument tray containing the following items:
– Large Rongeurs (2)
– Medium Rongeurs (2)
– Small Rongeurs (2)
– Scissors (4)
– Forceps (4)
– Mallet (1)
– Straight osteotome, 1 ¼”
5. Gauze
• Sterile Human Bone Marrow Processing Machine pack for BSC #1, containing the following items:
– Small rectangular stainless steel tray
– Oscillating grinder
– Stationary chamber
– Plunger
– Sterile Gauze
• Sterile Human Bone Marrow filter pack for BSC #2, containing the following items:
1. Stainless steel rack
2. #40 stainless steel sieve (425 μm)
3. #80 stainless steel sieve (180 μm)
4. Stainless steel sieve pan (2)
5. Stainless steel beaker (1)
6. Stainless steel paddle (2)
• Clean curtain around table
• Sterile translucent Nalgene jars (2)
• Sterile opaque Nalgene jar (2)
• Metal Paddle for spinning the bone chips (1)
• Sterile stainless steel pan (1)
• Sterile stainless steel bowl (1)
• Large plastic gray bin (1)
• Sterile autoclaved chain gloves (2 to 3 pairs)
D. Disposables (or equivalent)
• Sterile handwashing scrubs (sponges)
• Disinfectant of the month
• Sterile half-sheet drapes (4)
• Sterile 4×4 gauze
• Sterile Scoop
• Sterile conical tubes: 250 ml, 15 ml
• Sterile cryovials, 2 ml
• Sterile syringes: 10 ml, 60 ml
• Sterile needles, 18 gauge for blood products
• Sterile needles, 20-22 gauge for reagents
• Sterile Y-type blood set,170 micron filter (B. Braun Medical, Inc)
• Sampling site couplers
• Sterile disposable scalpels (12-15)
• Sterile pipettes: 1 ml, 10 ml, 25 ml, aspirating 5 ml
• Sterile glass beakers: 1 L, 600 ml
• Sterile surgical gowns, shoe covers, head cover, mask and face shield
• Sterile surgical gloves
• Ear plugs
• Sharps containers
• Red biohazard bags
• Vacuum collection flask with 2 associated sterile tubing sets
• Transport vials and media for anaerobic, aerobic and fungal cultures, and other designated transport containers for endotoxin testing supplied by Reference Laboratory (refer to relevant SOP, “Contaminant and Sterility Testing for Human Hematopoietic Products”).
E. Attachments
• SOP HCPF-001, Attachment I, ““Human Vertebral Body Marrow Processing Form”
• SOP HCPF-001, Attachment II, “Vertebral Body Marrow Summary Form”
• SOP HCPF-001, Attachment III, “Media Preparation Form: Bone Marrow Processing and Resuspension Media”
V. LIMITATIONS OR SPECIAL CONSIDERATIONS
1. If the final product is intended for transplant, a request for processing must be obtained by the cGMP Facility before product processing can begin, as described in relevant SOP, “Request for Processing: Cellular Products of Hematopoietic and Other Origin”. If recipient is unknown at the time the request form is received at the cGMP Facility, the form will be completed by the requesting physician, as soon as the intended recipient is selected.
2. DNAse (Dornase Alfa recombinant Pulmozyme) must be protected from light, and stored @ 2-8°C.
VI. PROCEDURE
A. Laboratory Preparation
1. Document the receipt of VBs from an OPO as described in the relevant SOP, “Receipt & Log of Human Organs, Tissue, Bone Marrow Blood & Blood Products”
2. Proper preparation for this procedure is essential to maintain sterility, and to allow the process to flow smoothly and expediciously. Therefore, all items used during processing, should be prepared and placed as indicated, prior to processing. However, media should not be removed from the refrigerator until it is needed.
3. For “Scrubbing & Gowning Procedure”, refer to relevant SOP.
4. Enter the clean room, Hematopoietic Cell Processing Facility, (HCPF) and perform the following tasks:
a. Prepare BSC #1, 2, 3 according to relevant SOP, “Use and Cleaning of Biological Safety Cabinets”. Insure the blowers are on in all BSC units.
b. Place two biohazard bags (place one bag inside another) in each large red waste bin, and a sharps container under each BSC.
NOTE: Double bagging must be done for safety purposes.
c. If the central vacuum system is not operational, assemble the vacuum collection system as follows:
– Attach one end of the vacuum tubing to the right side port of the pump and the other end to the “vac” valve of the collection flask.
– Attach one end of the second set of vacuum tubing to the collection flask at the “patient” opening, and squeeze the tubing at the other end into one of the metal slots at the side of the BSC.
d. Record manufacturers, lot numbers and expiration dates of all reagents, autoclavables, and disposables used on SOP HCPF-001, Attachment I, “Human Vertebral Body Marrow Processing Form”.
e. Obtain the following items and place them on a counter, for distribution to the appropriate areas, as described below:
– Sterile Human Bone Marrow Isolation Pack
– Sterile Human Bone Marrow Pack for filtering (in BSC #2)
– Sterile Human Bone Marrow Pack for processing machine (in BSC# 1) and all the items listed below in section IV. B.
– All items listed in section IV. C. “Disposables”
B. BSC & Bone Marrow Processing Machine Set Up
1. Remove the outer plastic bags from 4 sterile half sheets without touching the inner packaging; place one in each BSC, and one on the table.
2. In each BSC, place a sterile half sheet, so that the half sheet completely covers the sterile surface of the BSC (vented areas of BSC should remain uncovered).
3. Remove the curtain from its package, and hang it over the table by connecting the snap-on buttons.
4. After putting on another pair of sterile gloves, remove a half sheet from its inner packaging on the table and cover the table completely with it.
5. Open the outer wrapping of the bone marrow isolation pack. Open the inner (sterile) wrapping, and place the pack directly on the table.
6. Open the outer wrapping of the processing and the filter pack, and place it in BSCs #1 & 2, as described in the section C. Assemble the sieves so that the #40 sieve sits on top of the #80 sieve, with the sieve pan placed underneath.
7. Use the human bone marrow processing machine pack (BSC #1) to assemble the machine as follows:
a. Slide the oscillating grinder over the protruding column (line up the groove on the column with the matching space on the grinder) until it touches the base of the machine. Loosely tighten the grinder by turning the large round knob, located in the back of the machine counterclockwise.
b. Slide the chamber through the support bracket attached to the machine, so that the grooves interlock with those on the grinder (you may need to slide the grinder left or right on the arm, to allow the teeth to line up properly).
c. Loosely tighten each of the two black handles on the left side of the machine by turning them counterclockwise. This secures the mechanism to the base of the machine.
NOTE: These black handles are NOT sterile and, therefore, must be handled using sterile gauze.
d. Change gloves before proceeding to the next step.
Distribute the remaining items to the following areas (refer to the enclosed diagrams for the Set-up of the Table & BSC working areas):
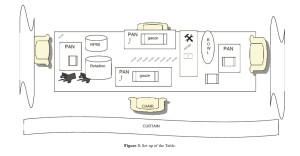
a. Table (Figure 3):
1. Translucent Nalgene jars (2)
2. Scalpels, disposable, sterile (12)
3. Syringe, 10 ml (1) with needle, 20 g (1)
4. Tubes for microbiology (aerobic, anaerobic, fungal)
5. Rectangular Pans (2-4)
6. Rongers & scissors
7. Sterile chain gloves
8. Sterile gauze, 4×4
b. BSC #1 (Figure 4):
1. Sterile scoop
2. Sterile gauze 4×4
3. Sterile bowls (2)
4. Bone Marrow Machine
5. Bone Marrow Machine Pack
6. Round Pan
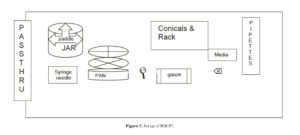
c. BSC #2 (Figure 5):
1. Nalgene jar, opaque (1)
2. Metal Paddle for the opaque Nalgene jar (1)
3. Metal beaker (1)
4. Round pan (1)
5. Sieve, # 40 (425 μm) (1)
6. Sieve, #80 (180 μm) (1)
7. Conical tubes, 250 ml (2 packs)
8. Sterile racks, 250 ml (3)
9. Sterile gauze 4×4
10. Sharpie Marker
11. Aspirating pipettes (20)
12. Pipettes, 10 ml (20)
13. Pipettes, 25 ml (3)
14. Pipettes, 1 ml (3)
15. Pipet aid, placed on the outer edge of the BSC (1)
16. Syringes, 10 ml (3)
17. Needles, 18 gauge (3) for blood products
18. Needles, 20 gauge (3) for reagents
19. Flask/beaker, 2 L (1)
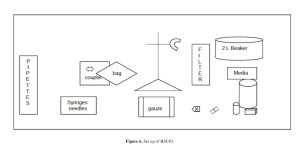
d. BSC#3 (Figure 6):
1. Sterile gauze, 4×4
2. Marker, Sharpie
3. Syringes, 10 ml (2)
4. Needles, 18 gauge (2) for blood products
5. Needles, 20 gauge (2) for reagents
6. Syringe, 60 ml (1)
7. Heparin, preservative-free for the final product (20 vials)
8. DNAse (bring DNAse out when ready to prepare the media) (4 vials)
9. Transfer bag, 2 L (1)
10. Y-type blood set (1)
11. Cobe coupler (1)
12. Ring stand (1)
13. Alcohol wipes
C. Media Preparation for Processing
1. Initiate SOP HCPF-001, Attachment III, “Bone Marrow Processing & Resuspension” form while preparing media.
2. Prepare media in BSC #3:
• Processing & Resuspension Media, prepare 5-7 x 1L bottles. Document on SOP HCPF-001, Attachment III.
For eah liter of media, add the following reagents:
a. RPMI-1640, 1L (Ca2+/Mg2+, phenol red free)
b. Human Serum Albumin, 25%, 100 ml
c. Heparin (10 ml of 1,000 U/ml vial or 1 ml of 10,000 U/ml vial)
d. Gentamycin, 40 mg/ml, 10 ml
e. HEPES,1 M, 2 ml
3. Heparin is added at a concentration of 10 units/ml, the bottle of media is 1,000 ml. Therefore, add 10,000 units of Heparin for every 1 L bottle of media.
NOTE: Preservative-free Heparin is supplied in a concentration of 1,000 units/ml.
4. Prepare 2 x 1L bottles of media, each containing one vial (2.5 mg) of DNAse.
NOTE: The concentration of DNAse is 1.0 mg/ml with 2.5 ml of solution per vial. Each vial, therefore, contains 2.5 mg of DNAse (refer to Section IV, above).
5. Prepare the other 3-5 1 L bottles of media using approximately two-
thirds of a DNAse vial or 1.66 ml of DNAse, i.e. each liter of media will contain ⅔ of a vial of DNAse.
6. Label all bottles of prepared media as soon as Heparin and DNAse are added. The label must include initials of the person preparing media, and the date of preparation and expiration.
D. Vertebral Body Cleaning
1. Aseptically, fill one jar placed on the table with approximately 500 ml of Betadine.
2. Aseptically, fill the other jar placed on the table with approximately 500 ml of RPMI-1640.
3. Add 500 ml of Processing/Resuspension Media containing Heparin and two vials of DNAse (2.5 ml), to the stainless steel bowl previously placed on the table.
4. Remove the bowl containing the jar with VBs from BSC and place it on the counter. Confirm that the UNOS number on the bag which holds the jar with VBs, matches the product label, donor chart, and the organ on-call sheet (refer to relevant SOP, “Donor Eligibility Criteria”) before proceeding. Confirm that all serology results are negative.
5. Open the bag containing the Nalgene jar with the media and VBs, and thoroughly wipe it using an alcohol wipe.
6. Transfer the Nalgene jar containing the VBs to the table.
WARNING: A face shield and a metal glove must be worn prior to VB cleaning and chopping. In addition, the use of earplugs is strongly recommended.
7. Obtain a sample for microbiological assessment from the VB procurement solution (Sx 1) as follows:
a. Remove the lid from the Nalgene jar containing the VBs
b. Using the 10 ml syringe with 20 g needle placed on the table earlier, withdraw a 10 ml sample (Sample #1) from the procurement media. Refer to relevant SOP, “Contaminant and Sterility Testing for Hematopoietic Products”.
8. Wash the VBs in Betadine and RPMI-1640 as follows:
a. Remove VBs from the Nalgene jar and immerse them in the jar containing Betadine for 3-5 seconds, but not longer.
b. Remove VBs from Betadine; transfer and wash them in the jar containing RPMI-1640. If a significant amount of Betadine is still left clinging to a VB, rinse again in fresh RPMI-1640.
c. Keep VBs in RPMI-1640, completely immersed, until they are processed. Do not allow them to dry.
9. Put on a sterile chain glove over sterile disposable gloves on the opposite hand you use to cut with.
10. Use a scalpel to separate vertebral bodies in the column by cutting through the intervertebral discs. Discard scalpels in the sharps container after use.
11. Place an individual VB on a piece of sterile gauze.
12. Use a scalpel to remove the intervertebral disc on each side of a VB.
13. Use scissors to aid in the removal of the disc, especially around the perimeter of the VB.
14. Use a scalpel, ronguers and/or scissors to remove any fat, muscle, and connective tissue on and around the VB. Do not let VBs dry out, always return them to the liquid after cleaning.
15. Count the number of VBs after they are cleaned, and enter this data on SOP HCPF-001, Attachment I, “Human Vertebral Body Marrow Processing Form”.
16. Once VBs are cleaned, they must be chopped into smaller pieces. Use the osteotome and mallet to chop each VB into approximate 1 cm2 pieces.
17. Place all the pieces in the stainless steel bowl containing Processing/Resuspension media with Heparin and DNAse.
18. Place the Nalgene jar on the balance and tare. Transfer the VB pieces from the stainless steel bowl to the pre-tared jar placed on the balance, and weigh. Record the data on SOP HCPF-001, Attachment I.
NOTE: Weigh only VBs, not the media in the bowl.
19. Add approximately 300 ml of Processing media to the stainless steel bowl, located in BSC #1, and add chopped VBs to the bowl.
E. Automated Chopping of Vertebral Bodies (VB)
1. Using a piece of sterile gauze, turn the bone marrow machine on. The speed should be set to 50-60.
2. Place the stainless steel sieve bowl under the oscillating grinder to catch the chips as they are being processed.
3. Place 4-5 VB pieces into the chamber at a time.
4. Pieces will usually be ground automatically: when this does not happen, use the plunger to push the pieces through the grinder. Do not apply constant force on the plunger. Allow the pieces to move freely from time to time.
5. If pieces become lodged in the teeth of the grinder, turn the machine off and try to clear chamber with scissors.
6. Make sure processed chips are immersed in Processing/Resuspension Media (this media contains Heparin and 2.5 mg of DNAse). Use the sterile scoop to evenly distribute the chips throughout the sieve pan.
7. Once all VB pieces are processed, thoroughly rinse the chamber and transfer the bowl containing the bone chips to BSC #2.
F. VB Processing
1. Aseptically, pour the Processing/Resuspension media that contains the bone chips into the #80 sieve using sterile technique.
2. After all media is passed through the sieve assembley, rinse VB chips using additional Processing media and, aseptically, transfer everything from the sieve pan to a stainless steel beaker. Place the sieve with the bone chips on an empty round pan to collect residual media.
3. Aseptically, pour the collected media from the stainless steel beaker into 250ml conicals using sterile technique, and label each conical using a in-process label (refer to relevant SOP, “Labeling of Hematopoietic Products”).
4. Shake the suspension, in order to release cells trapped within the trabecular network of the VBs, as follows:
a. Prepare 2 opaque Nalgene jars. After placing the sterile metal paddle in each, transfer the bone chips from the #40 sieve into the jar, equally deviding the chips between the two jars.
b. Fill each jar ¾ full with Processing/Resuspension media (media prepared with 1.66 mg of DNAse) and close the lid tightly.
c. Label each jar using a partial label (refer to relevant SOP, “Labeling of Hematopoietic Products”).
d. Transfer each jar to the shaking machine and secure it.
e. Flipping the two switches located in the back of the machine, turn the machine on and begin shaking (one switch controls the rotation, the other vertical motion)
f. Allow the bone chips to shake for 30 minutes (1st Wash).
5. Centrifuge filtered cells while the bone chips are shaking as follows:
a. Place the 250 ml conicals into the centrifuge.
b. Centrifuge at 280 x g (1,300 rpm) for 10 minutes, at 16-20°C.
6. Aspirate the supernatant as follows:
a. Place the 250 ml conicals containing centrifuged cells into BSC #2.
b. Attach the free end of the vacuum tubing to an end of an aspirating pipette.
c. Open each conical, turn on the vacuum pump and aspirate the supernatant from each tube. To avoid loosing the cells during aspiration, do not aspirate too closely to the pellet.
7. Homogeneously resuspend each pellet left in each 250 ml conical after aspiration, as follows:
a. Swirl the conical by hand to loosen the pellet.
b. Use a sterile 10 ml pipette attached to a Pipet-aid to resuspend the pellet by pipeting the cells up and down gently until the cells are homogeneously resuspended.
c. Add ~10 ml of media to each conical. Using a sterile, disposable 3ml transfer pipette, resuspend the cells until a single-cell suspension is achived, by pipetting cells up and down.
8. Transfer the cells from all the conicals to a new sterile 250 ml conical and label using a partial label (refer to relevant SOP, “Labeling of Hematopoietic Products”).
9. Rinse the conicals, with Processing/Resuspension media to make sure all the cells are recovered. Add this rinse to a new conical already containing the cells.
10. Fill the new conical containing the cells with Processing/Resuspension media. This conical can be left in the BSC at room temperature until all washes are complete.
11. While the bone chips are shaking, clean up soiled items as follows:
a. Remove all HBM numbers from Nalgene jars.
b. Disassemble the machine by reversing the order of the assembly instructions (section VI.B.5) and transfer the stainless steel dirty supplies and instruments to the dirty area, in a bin containing hot water and Haemosol or equivalent.
12. At the end of the first wash (30 minutes), stop both bone marrow shakers. Repeat steps 1-10 of this section using fresh media and chips from the 1st wash. Centrifuge the supernatant from the 1st Wash at 280 x g (1,300 rpm) for 10 minutes at 16-20°C.
13. Allow the bone chips to shake for another 30 minutes (2nd Wash).
14. Clean the materials in BSC #2 as follows:
a. Discard the bone chips, unless they are required for research (the fact that donor tissue (VB) has research consent must be confirmed prior to distribution).
b. Aspirate fluid and remove fat tissue with gauze.
c. Place the sieves, sieve pan, stainless steel beaker, and opaque Nalgene jar in a large gray bin with the other dirty materials.
15. Quick spin the conicals containing the cells to remove any bone fragments and debris as follows:
a. Resuspend the cells by pipetting up and down using a 10 ml pipette first, followed by a 3 ml transfer pipetter.
b. Place the tubes in the centrifuge with the brake “OFF” and press “START”.
c. When the centrifuge reaches 275 x g (1,000 rpm) at 16-20°C, immediately press “STOP”.
16. Immediately after the quick spin, poor the supernatant from the conical into a 2 liter flask, placed previously in BSC #2. After the quick spin, the cells remain in suspension, while the heavier bone fragments and debris form a very loose pellet.
17. In BSC #3, using the Y-type blood set (170 μm filter size) to further remove any bone spicules4 or clumps, filter the product as described below:
NOTE: The pore size of the Y-type blood set used is large enough (170μm) to prevent any mononuclear cell loss, as indicated by counting the product before and after filtration (unpublished data).
a. Insert the COBE coupler into the side port of the 2 L transfer bag.
b. Aseptically remove the plunger from a 60 ml syringe and attach the 60 ml syringe into the clamp of the ring stand.
c. Attach the COBE coupler to the syringe by turning the coupler counterclockwise.
d. Pipette the cell suspension from from the 2 L flask to the 2 L transfer bag.
e. When all the cells are transferred into the 2 L transfer bag, close the clamp on the COBE coupler and remove it from the syringe.
f. Seal off one end of the Y-type blood set using the heat sealer. Insert the other end of the Y-type blood set into the central port of the transfer bag.
g. Ensure that all valves of the Y-type blood set are closed.
h. Connect Y-type blood set to the 2 L bag, and the other end of the filter goes to a new 1 L beaker prepared to receive the final filtered product.
i. Hang the 2 L bag containing the product from the outside of the BSC #3 to filter the cells by gravity.
j. When all the cells are filtered, use the heat sealer to remove the bag from the filter set as described in relevant SOP, “Use, Quality Control and Maintenance of the Heat Sealer”.
k. Record the product volume.
18. Samples of the filtered product are taken for (a total of 4-5 ml of cells is required):
a. Sterility testing (refer to relevant SOP, “Sterility Testing”). Remove a total of 3 ml: 1 ml for each, anerobic, aerobic and fungal culture from the supernatant.
NOTE: Gram stain is not performed unless the product is transplanted immediately following processing (fresh).
b. Nucleated cell counts (refer to relevant SOPs, “Use Quality Control and Maintenance of the Coulter© T diff™ Analyser” and “Manual Cell Countr and Differential”). Remove 100 μl of the cell suspension.
c. Cell viability (refer to relevant SOP, “Trypan Blue Viability Testing”). Use the rest of the 100 μl counting sample to estimated cell viability.
d. Hematopoietic progenitor cell assay (refer to relevant SOP, “Hematopoietic Progenitor Cell Assay”), if requested. Remove 0.5 ml of the cell suspension for this assay.
e. Flow Cytometric Analysis (Refer to relevant SOP and Form). Remove 0.5 ml of the cell suspension for this assay.
19. Viability is also performed by Flow Cytometry using 7AAD antibody, while the function of the preparation is assessed by “Hematopoietic Progenitor Cell Assay” described in relevant SOP (when requested by the requesting physician/Responible Head).
G. Vertebral Body Bone Marrow Product Final Distribution
1. For labeling instructions refer to relevant SOP.
2. There are several possible final dispositions for the product. It can be
a. Transplanted immediately following processing, or
b. Cryopreserved, or
c. Further processed to obtain CD34+ve cells, and then cryopreserved/transplanted
d. Designated for information purposes
3. If the cells are designted to be transplanted after processing and must be stored (not to ecceed 12 hours) for any reason, store the cell suspension in the Processing/Resuspention media which contains 5%HSA, at 2-8°C (Attachment III of this SOP). Just before transplant, the product must be washed and resuspended in the Transplant media containing 2.5% HSA (refer to relevant SOP).
4. If cells are to be cryopreserved, follow the relevant SOP, “Cryopreservation of Hematopoietic Products”.
5. For further processing to enrich for selected cell subpopulations follow the relevant SOPs (e.g., CD34+, MSCs, etc.)
6. Document the final disposition of the product on the appropriate SOP
H. Cleanup of the Laboratory
Clean up the laboratory following the relevant SOP, “Decontamination of Laboratory Work Areas and Equipment”.
1. Disconnect and discard disposable sections of the vacuum collection system.
2. Discard any opened, unused media or transport to research area with bottles clearly labeled for “Research Only”.
3. Close all sharps containers and secure red biohazard bags according to the SOP on “Disposal of Waste Materials” and remove from the laboratory.
4. Remove all soiled supplies from the cleanroom.
I. Quality Control
All equipment used will be maintained according to the SOP on “Cleaning, Maintenance and Quality Control of Equipment”.
J. Bone Marrow Summary Data
1. When the processing is complete, print and file Attachment II of this SOP in the corresponding batch record.
2. Complete all the other Attachments of this procedure and include them in the corresponding batch record.
VII. RECORD REVIEW
1. All Attachments must be reviewed for completeness and accuracy.
2. Records will be reviewed by the Operations Director or Designee as per the relevant SOP: “Supervisory Review of Records & Procedures”.
VIII. RECORD RETENTION
Records will be maintained in the Records Room following the time outlined in the relevant SOP: “Record Retention and Archival System”.
References
- Fontes PA, Ricordi C, Rao AS, Rybka WB, Dodson SF, Broznick B, et al. Human Vertebral Bodies as a Source of Bone Marrow for Cell Augmentation in Whole Organ Allografts. In: Ricordi C, editor. Methods in Cell Transplantation. Austin, TX, USA: R.G. Landes Company; 1995. pp. 619-628. (back)
- Ricordi C, Tzakis AG, Demetris AJ, Zeevi A, Rybka WB, Nalesnik MA, et al. Reversal of graft-versus-host disease with infusion of stored autologous bone marrow cells following combined liver-bone marrow allotransplantation in man. Transplant Science 1993; 3(1): 76-77. (back)
- Carroll PB, Fontes P, Rao AS, Ricordi C, Rilo HL, Zeevi A, et al. Simultaneous solid organ, bone marrow, and islet allotransplantation in type I diabetic patients. Transplant Proc 1994; 26(6): 3523-3524. (back)
- Fontes P, Rao AS, Demetris AJ, Zeevi A, Trucco M, Carroll P, et al. Bone marrow augmentation of donor-cell chimerism in kidney, liver, heart, and pancreas islet transplantation. Lancet 1994; 344(8916): 151-155. (back)
- Fontes P, Rao AS, Ricordi C, Rybka WB, Dodson FS, Broznick B, et al. Human bone marrow obtained from vertebral bodies: cell isolation, phenotyping, progenitor assay, and transplantation. Transplant Proc 1994; 26(6): 3406-3407. (back)
- Fontes P, Rao AS, Ricordi C, Zeevi A, Kocova M, Rybka WB, et al. Human-to-baboon bone marrow transplantation after conditioning with nonlethal irradiation. Transplant Proc 1994; 26(6): 3367-3368. (back)
- Ricordi C, Murase N, Rastellini C, Behboo R, Demetris AJ, Starzl TE. Donor bone marrow cell infusion without recipient cytoablation induces acceptance of rat islet allografts. Transplant Proc 1994; 26(6): 3358. (back)
- Ricordi C, Tzakis AG, Zeevi A, Rybka WB, Demetris AJ, Fontes PA, et al. Reversal of graft-versus-host disease with infusion of autologous bone marrow. Cell Transplant 1994; 3(2): 187-192. (back)
- Bottino R, Linetsky E, Selvaggi G, Kong SS, Qian T, Ricordi C. Automation of human vertebral body bone marrow isolation. Ann N Y Acad Sci 1995; 770: 364-365. (back)
- Bottino R, Linetsky E, Selvaggi G, Kong SS, Qian T, Ricordi C. Human vertebral body bone marrow harvest: comparison between manual and automated methods. Transplant Proc 1995; 27(6): 3340. (back)
- Burke GW, Ricordi C, Karatzas T, Carreno M, Cirocco R, Ciancio G, et al. Donor bone marrow infusion in simultaneous pancreas/kidney transplant recipients: a preliminary study. Transplant Proc 1995; 27(6): 3121-3122. (back)
- Kenyon NS, Xu XM, Garcia-Serra A, Ricordi C. Immunogenicity and stem cell content of class II depleted human vertebral body bone marrow. Ann N Y Acad Sci 1995; 770: 361-363. (back)
- Kenyon NS, Xu XM, Garcia-Serra A, Ricordi C. Effect of depletion of class II bright cells on the immunogenicity and stem cell content of human vertebral body bone marrow. Transplant Proc 1995; 27(6): 3419. (back)
- Kenyon NS, Xu XM, Knapp J, Selvaggi GS, Bottino R, Kong SS, et al. Automated processing of human vertebral body bone marrow yields preparations with stem cell content similar to that obtained with traditional manual preparation. Transplant Proc 1995; 27(6): 3418. (back)
- Kong SS, Kenyon NS, Brendel M, Tzakis AG, Miller J, Ricordi C. Effect of preservation conditions on human vertebral body marrow. Transplant Proc 1995; 27(6): 3415. (back)
- Kong SS, Selvaggi G, Kenyon N, Bottino R, Linetsky E, Ricordi C. A simple method for depletion of bone fragments from human vertebral body marrow. Transplant Proc 1995; 27(6): 3417. (back)
- Kong SS, Selvaggi G, Kenyon N, Knapp J, Olson L, Tzakis AG, et al. Suitability of neonatal vertebral body marrow for transplant applications. Transplant Proc 1995; 27(6): 3416. (back)
- Ricordi C, Karatzas T, Selvaggi G, Neri J, Fernandez H, Ruiz MP, et al. Enhanced allograft acceptance by multiple infusions of donor bone marrow in humans. Transplant Proc 1995; 27(6): 3381. (back)
- Ricordi C, Karatzas T, Selvaggi G, Nery J, Webb M, Fernandez H, et al. Multiple bone marrow infusions to enhance acceptance of allografts from the same donor. Ann N Y Acad Sci 1995; 770: 345-350. (back)
- Rybka WB, Fontes PA, Rao AS, Winkelstein A, Ricordi C, Ball ED, et al. Hematopoietic progenitor cell content of vertebral body marrow used for combined solid organ and bone marrow transplantation. Transplantation 1995; 59(6): 871-874. (back)
- Ciancio G, Carreno M, Mathew J, Ricordi C, Garcia R, Karatzas T, et al. Human donor bone marrow cells can enhance hyporeactivity in renal transplantation using maintenance FK 506 and OKT3 induction therapy. Transplant Proc 1996; 28(2): 943-944. (back)
- Fernandez LA, Romaguera R, Viciana AL, Ruiz P, Tzakis AG, Ricordi C. Pulmonary embolism with bone fragments following vertebral body marrow infusion for tolerance induction. Cell Transplant 1996; 5(4): 513-516. (back)
- Garcia-Morales R, Esquenazi V, Zucker K, Gomez CI, Fuller L, Carreno M, et al. An assessment of the effects of cadaver donor bone marrow on kidney allograft recipient blood cell chimerism by a novel technique combining PCR and flow cytometry. Transplantation 1996; 62(8): 1149-1160. (back)
- Tzakis A, Webb M, Nery J, Rogers A, Koutouby R, Ruiz P, et al. Experience with intestinal transplantation at the University of Miami. Transplant Proc 1996; 28(5): 2748-2749. (back)
- Weppler D, Khan R, Fragulidis GP, Nery JR, Ricordi C, Tzakis AG. Status of liver and gastrointestinal transplantation at the University of Miami. Clin Transpl 1996: 187-201. (back)
- Alejandro R, Ricordi C, Angelico MC, Nery J, Webb M, Fernandez L, et al. Donor bone marrow infusions in conjunction with liver-islet allotransplantation in patients with type 2 diabetes. Transplant Proc 1997; 29(1-2): 745. (back)
- Burke GW, Ricordi C, Karatzas T, Carreno M, Markou M, Cirocco R, et al. Donor bone marrow infusion in simultaneous pancreas/kidney transplantation with OKT3 induction: evidence for augmentation of chimerism. Transplant Proc 1997; 29(1-2): 1207-1208. (back)
- Fontes P, Rogers J, Rao AS, Trucco M, Zeevi A, Ricordi C, et al. Evidence for engraftment of human bone marrow cells in non-lethally irradiated baboons. Transplantation 1997; 64(11): 1595-1598. (back)
- Garcia-Morales R, Carreno M, Mathew J, Zucker K, Cirocco R, Ciancio G, et al. The effects of chimeric cells following donor bone marrow infusions as detected by PCR-flow assays in kidney transplant recipients. J Clin Invest 1997; 99(5): 1118-1129. (back)
- Garcia-Morales R, Esquenazi V, Carreno M, Karatzas T, Gomez C, Cirocco R, et al. PCR-flow analysis used to detect the levels of chimerism in peripheral blood of bone-marrow infused organ allograft recipients at the time of rejection episodes. Transplant Proc 1997; 29(4): 2179-2180. (back)
- Garcia-Morales R, Esquenazi V, Zucker K, Gomez CI, Fuller L, Carreno M, et al. Assessment of the effects of cadaveric donor bone marrow on chimerism in kidney transplant recipients by the polymerase chain reaction-flow technique. Transplant Proc 1997; 29(1-2): 1219-1221. (back)
- Kenyon NS, Russell TR, Xu XM, Knapp J, Ricordi C. Enrichment of hematopoietic stem cells from human vertebral body marrow. Transplant Proc 1997; 29(4): 1951. (back)
- Knapp JC, Kenyon NS, Russell TR, Ricordi C. An automated method for flow-cytometric analysis of human vertebral body marrow. Transplant Proc 1997; 29(4): 1953. (back)
- Kong SS, Knapp J, Selvaggi G, Kenyon NS, Ricordi C. Characterization of pediatric vertebral bone marrow. Transplant Proc 1997; 29(4): 1955. (back)
- Kong SS, Ricordi C, Inverardi L. Possible role of preformed natural antibodies in preventing bone marrow engraftment in a discordant xenogeneic species combination (human-to-mouse). Transplant Proc 1997; 29(4): 2069-2070. (back)
- Linetsky E, Kenyon N, Li H, Xu X, Ricordi C. Increased immunogenicity of human vertebral body marrow after processing in bovine versus human serum albumin. Transplant Proc 1997; 29(4): 1960. (back)
- Linetsky E, Li H, Fernandez L, Bottino R, Lehmann R, Selvaggi G, et al. Variables affecting human vertebral body marrow yields. Transplant Proc 1997; 29(4): 1959. (back)
- Mathew JM, Carreno M, Fuller L, Ricordi C, Tzakis A, Esquenazi V, et al. Modulatory effects of human donor bone marrow cells on allogeneic cellular immune responses. Transplantation 1997; 63(5): 686-692. (back)
- Olson LC, Ricordi C, Karatzas T, Ciancio G, Waters JD, Burke GW, et al. Vertebral body procurement from multiorgan donors for bone marrow harvest. Transplant Proc 1997; 29(4): 2243-2245. (back)
- Ricordi C, Alejandro R, Angelico MC, Fernandez LA, Nery J, Webb M, et al. Human islet allografts in patients with type 2 diabetes undergoing liver transplantation. Transplantation 1997; 63(3): 473-475. (back)
- Ricordi C, Karatzas T, Nery J, Webb M, Fernandez L, Selvaggi G, et al. Effect of timing of donor bone marrow infusions on liver allograft survival. Transplant Proc 1997; 29(1-2): 1186. (back)
- Ricordi C, Karatzas T, Nery J, Webb M, Selvaggi G, Fernandez L, et al. High-dose donor bone marrow infusions to enhance allograft survival: the effect of timing. Transplantation 1997; 63(1): 7-11. (back)
- Tsaroucha AK, Ricordi C, Noto TA, Kenyon NS, Garcia-Morales R, Nery JR, et al. Donor peripheral blood stem cell infusions in recipients of living-related liver allografts. Transplantation 1997; 64(2): 362-364. (back)
- Burke GW, Ciancio G, Garcia-Morales R, Ricordi C, Alejandro R, Roth D, et al. Higher percentage of donor CD 34+ expression in peripheral blood of simultaneous pancreas/kidney/donor bone marrow versus than kidney/islet cell/donor bone marrow recipients. Transplant Proc 1998; 30(2): 535-536. (back)
- Burke GW, Ciancio G, Garcia-Morales R, Ricordi C, Alejandro R, Roth D, et al. Evidence for microchimerism in peripheral blood, bone marrow, and skin following donor bone marrow/kidney-pancreas transplantation at 3 years. Transplant Proc 1998; 30(4): 1555. (back)
- Carreno MR, Esquenazi V, Gomez C, Garcia-Morales R, Mathew J, Cirocco R, et al. Immunophenotyping and cellular immune responses of cadaveric donor bone marrow cells. Transplant Proc 1998; 30(3): 727-728. (back)
- Ciancio G, Garcia-Morales R, Burke GW, Roth D, Esquenazi V, Rosen A, et al. Donor bone marrow infusion in renal transplantation. Transplant Proc 1998; 30(4): 1365-1366. (back)
- Garcia-Morales R, Carreno M, Mathew J, Cirocco R, Zucker K, Ciancio G, et al. Continuing observations on the regulatory effects of donor-specific bone marrow cell infusions and chimerism in kidney transplant recipients. Transplantation 1998; 65(7): 956-965. (back)
- Mathew JM, Carreno M, Zucker K, Fuller L, Kenyon N, Esquenazi V, et al. Cellular immune responses of human cadaver donor bone marrow cells and their susceptibility to commonly used immunosuppressive drugs in transplantation. Transplantation 1998; 65(7): 947-955. (back)
- Mathew JM, Carreno M, Zucker K, Fuller L, Vallone T, Ricordi C, et al. Differential resistance of the cellular immune responses and immunoregulatory properties of human cadaveric donor bone marrow cells to immunosuppressive drugs commonly used in transplantation. Transplant Proc 1998; 30(4): 1073-1074. (back)
- Angelico MC, Alejandro R, Nery J, Webb M, Bottino R, Kong SS, et al. Transplantation of islets of Langerhans in patients with insulin-requiring diabetes mellitus undergoing orthotopic liver transplantation—the Miami experience. J Mol Med (Berl) 1999; 77(1): 144-147. (back)
- Chatzipetrou MA, Mathew JM, Kenyon NS, Esquenazi V, Miller J, Ricordi C, et al. Analysis of post-transplant immune status in recipients of liver/bone marrow allografts. Human Immunol 1999; 60(12): 1281-1288. (back)
- D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Mineral Res 1999; 14(7): 1115-1122. (back)
- Mathew JM, Carreno M, Fuller L, Ricordi C, Kenyon N, Tzakis AG, et al. In vitro immunogenicity of cadaver donor bone marrow cells used for the induction of allograft acceptance in clinical transplantation. Transplantation 1999; 68(8): 1172-1180. (back)
- Miller J, Mathew J, Garcia-Morales R, Zucker KE, Carreno M, Jin Y, et al. The human bone marrow as an immunoregulatory organ. Transplantation 1999; 68(8): 1079-1090. (back)
- Pinna AD, Weppler D, Berho M, Masetti M, DeFaria W, Kato T, et al. Unusual presentation of graft-versus-host disease in pediatric liver transplant recipients: evidence of late and recurrent disease. Pediatr Transplant 1999; 3(3): 236-242. (back)
- Jin Y, Fuller L, Esquenazi V, Blomberg BB, Rosen A, Tzakis AG, et al. Bone marrow cells inhibit the generation of autologous EBV-specific CTL. Human Immunol 2000; 61(6): 538-547. (back)
- Mathew JM, Fuller L, Carreno M, Garcia-Morales R, Burke GW, 3rd, Ricordi C, et al. Involvement of multiple subpopulations of human bone marrow cells in the regulation of allogeneic cellular immune responses. Transplantation 2000; 70(12): 1752-1760. (back)
- Mathew JM, Garcia-Morales R, Fuller L, Rosen A, Ciancio G, Burke GW, et al. Donor bone marrow-derived chimeric cells present in renal transplant recipients infused with donor marrow. I. Potent regulators of recipient antidonor immune responses. Transplantation 2000; 70(12): 1675-1682. (back)
- Ciancio G, Garcia-Morales R, Mathew J, Carreno M, Burke GW, Ricordi C, et al. Donor bone marrow infusions are tolerogenic in human renal transplantation. Transplant Proc 2001; 33(1-2): 1295-1296. (back)
- Ciancio G, Miller J, Garcia-Morales RO, Carreno M, Burke GW, 3rd, Roth D, et al. Six-year clinical effect of donor bone marrow infusions in renal transplant patients. Transplantation 2001; 71(7): 827-835. (back)
- Garcia-Morales RO, Ciancio G, Mathew J, Jin Y, Rosen A, Ricordi C, et al. Perioperative donor bone marrow infusion in cadaver kidney transplant recipients. Transplant Proc 2001; 33(7-8): 3840-3843. (back)
- Mathew JM, Fuller L, Carreno M, Burke GW, Ciancio G, Ricordi C, et al. Donor bone marrow cells used for the induction of allograft acceptance in clinical transplantation as regulators of autologous immune responses. Transplant Proc 2001; 33(1-2): 108-109. (back)
- Mathew JM, Garcia-Morales R, Fuller L, Rosen A, Burke GW, Ciancio G, et al. Cells of donor phenotype present in renal transplant recipients infused with donor marrow: potent regulators of antidonor immune responses of the recipient. Transplant Proc 2001; 33(1-2): 127-128. (back)
- Ciancio G, Burke GW, Garcia-Morales R, Suzart K, Rosen A, Ricordi C, et al. Effect of living-related donor bone marrow infusion on chimerism and in vitro immunoregulatory activity in kidney transplant recipients. Transplantation 2002; 74(4): 488-496. (back)
- Garavito G, Klein D, Denis M, Pugliese A, Ricordi C, Pastori RL. Real-time sequence-specific primer polymerase chain reaction amplification of HLA class II alleles: a novel approach to analyze microchimerism. Transplantation 2002; 73(5): 822-825. (back)
- Jin Y, Fuller L, Carreno M, Esquenazi V, Blomberg BB, Wei YT, et al. Functional and phenotypic properties of peripheral T cells anergized by autologous CD3(+) depleted bone marrow cells. Human Immunol 2002; 63(7): 567-575. (back)
- Mathew JM, Carreno M, Fuller L, Burke GW, 3rd, Ciancio G, Ricordi C, et al. Regulation of alloimmune responses (GvH reactions) in vitro by autologous donor bone marrow cell preparation used in clinical organ transplantation. Transplantation 2002; 74(6): 846-855. (back)
- Carreno MR, Fuller L, Mathew JM, Ciancio G, Burke GW, Esquenazi V, et al. Human donor bone marrow cells induce in vitro “suppressor T cells” that functionally suppress autologous B cells. Human Immunol 2003; 64(1): 21-30. (back)
- Mathew JM, Blomberg B, Fuller L, Burke GW, Ciancio G, Kenyon N, et al. A novel micro-cell-mediated lympholytic assay for the evaluation of regulatory cells in human alloreactive CTL responses. J Immunol Methods 2003; 272(1-2): 67-80. (back)
- Mathew JM, Garcia-Morales RO, Carreno M, Jin Y, Fuller L, Blomberg B, et al. Immune responses and their regulation by donor bone marrow cells in clinical organ transplantation. Transpl Immunol 2003; 11(3-4): 307-321. (back)
- Jin Y, Fuller L, Ciancio G, Burke GW, 3rd, Tzakis AG, Ricordi C, et al. Antigen presentation and immune regulatory capacity of immature and mature-enriched antigen presenting (dendritic) cells derived from human bone marrow. Human Immunol 2004; 65(2): 93-103. (back)
- Pileggi A, Ricordi C, Kenyon NS, Froud T, Baidal DA, Kahn A, et al. Twenty years of clinical islet transplantation at the Diabetes Research Institute—University of Miami. Clin Transplant 2004: 177-204. (back)
- Jin Y, Fuller L, Rosen A, Ciancio G, Burke GW, 3rd, Ricordi C, et al. Campath-1H does not alter bone marrow cell regulatory function. Human Immunol 2005; 66(6): 637-643. (back)
- Tryphonopoulos P, Tzakis AG, Weppler D, Garcia-Morales R, Kato T, Madariaga JR, et al. The role of donor bone marrow infusions in withdrawal of immunosuppression in adult liver allotransplantation. Am J Transplant 2005; 5(3): 608-613. (back)
- Cirocco RE, Carreno MR, Mathew JM, Garcia-Morales RO, Fuller L, Esquenazi V, et al. FoxP3 mRNA transcripts and regulatory cells in renal transplant recipients 10 years after donor marrow infusion. Transplantation 2007; 83(12): 1611-1619. (back)
- Jin Y, Fuller L, Esquenazi V, Blomberg BB, Burke GW, 3rd, Ciancio G, et al. Induction of auto-reactive regulatory T cells by stimulation with immature autologous dendritic cells. Immunol Invest 2007; 36(2): 213-232. (back)
- Mathew JM, Blomberg B, Ricordi C, Esquenazi V, Miller J. Evaluation of the tolerogenic effects of donor bone marrow cells using a severe combined immunodeficient mouse-human islet transplant model. Human Immunol 2008; 69(10): 605-613. (back)
- Fotino C, Ricordi C, Lauriola V, Alejandro R, Pileggi A. Bone marrow-derived stem cell transplantation for the treatment of insulin-dependent diabetes. Rev Diabet Stud 2010; 7(2): 144-157. (back)
- Mathew JM, Ciancio G, Burke GW, Garcia-Morales RO, Rosen A, Wang E, et al. Immune “tolerance profiles” in donor bone marrow infused kidney transplant patients using multiple ex vivo functional assays. Human Immunol 2010; 71(6): 566-576. (back)
- Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA 2012; 307(11): 1169-1177. (back)
- Luo JZ, Xiong F, Al-Homsi AS, Ricordi C, Luo L. Allogeneic bone marrow cocultured with human islets significantly improves islet survival and function in vivo. Transplantation 2013; 95(6): 801-809. (back)
- D’Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci 2004; 117(Pt 14): 2971-2981. (back)
- Huang CY, Reuben PM, D’Ippolito G, Schiller PC, Cheung HS. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec A Discov Mol Cell Evol Biol 2004; 278(1): 428-436. (back)
- D’Ippolito G, Howard GA, Roos BA, Schiller PC. Isolation and characterization of marrow-isolated adult multilineage inducible (MIAMI) cells. Exp Hematol 2006; 34(11): 1608-1610. (back)
- Tatard VM, D’Ippolito G, Diabira S, Valeyev A, Hackman J, McCarthy M, et al. Neurotrophin-directed differentiation of human adult marrow stromal cells to dopaminergic-like neurons. Bone 2007; 40(2): 360-373. (back)
- Rahnemai-Azar A, D’Ippolito G, Gomez LA, Reiner T, Vazquez-Padron RI, Perez-Stable C, et al. Human marrow-isolated adult multilineage-inducible (MIAMI) cells protect against peripheral vascular ischemia in a mouse model. Cytotherapy 2011; 13(2): 179-192. (back)
- Fontes P, Rao AS, Ricordi C, Zeevi A, Kocova M, Rybka WB, et al. Human-to-baboon bone marrow transplantation after conditioning with nonlethal irradiation. Transplant Proc 1994; 26(6): 3367-3368. (back)
- Brendel MD, Kong SS, Schachner RD, Qian T, Selvaggi G, Alejandro R, et al. Canine islet allograft survival after donor specific vertebral body derived bone marrow cell transplantation without irradiation conditioning of the recipient. Transplant Proc 1995; 27(6): 3174. (back)
- Brendel MD, Kong SS, Schachner RD, Qian T, Selvaggi G, Alejandro R, et al. The influence of donor specific vertebral body derived bone marrow cell infusion on canine islet allograft survival without irradiation conditioning of the recipient. Exp Clin Endocrinol Diabetes 1995; 103(Suppl 2): 129-132. (back)
- Kenyon NS, Knapp JC, Xu XM, Selvaggi G, Fernandez L, McMannis J, et al. Characterization of baboon vertebral body marrow. Transplant Proc 1997; 29(4): 1952. (back)
- Kenyon NS, Selvaggi G, Fernandez L, Xu XM, Knapp J, Montelongo J, et al. Infusion of class II DIM donor bone marrow enhances islet allograft survival in low-dose CyA treated dogs. Transplant Proc 1997; 29(4): 2189. (back)
- Han D, Ricordi C, Kenyon NS. Establishment of a method for analysis of chimerism in a baboon model (Papio hamadryas) of islet/bone marrow transplantation. Transplant Proc 1998; 30(2): 554-555. (back)
- Spada M, Alessiani M, Bonfichi M, Viezzoli A, Pileggi A, Abbiati F, et al. Development of a model of combined bone marrow and small bowel transplantation in swine. Transplant Proc 1998; 30(6): 2609-2610. (back)
- Alessiani M, Spada M, Bonfichi M, Ferrari P, Abbiati F, Arbustini E, et al. Effect of perioperative donor bone marrow infusion after small bowel transplantation in swine: preliminary results. Transplant Proc 1998; 30(6): 2577-2578. (back)
- Han D, Ricordi C, Xu X, Kenyon NS. Quantitative polymerase chain reaction assessment of chimerism in non-human primates after sex-mismatched islet and bone marrow transplantation. Transplantation 2000; 69(8): 1717-1721. (back)
- Comite P, Cobianchi L, Avanzini MA, Mantelli M, Achille V, Zonta S, et al. Immunomodulatory properties of porcine, bone marrow-derived multipotent mesenchymal stromal cells and comparison with their human counterpart. Cell Mol Biol (Noisy-le-grand) 2011; 57(Suppl): OL1600-1605. (back)
- Olson LC, Ricordi C, Karatzas T, Ciancio G, Waters JD, Burke GW, et al. Vertebral body procurement from multiorgan donors for bone marrow harvest. Transplant Proc 1997; 29(4): 2243-2245. (back)
- Linetsky E, Li H, Fernandez L, Bottino R, Lehmann R, Selvaggi G, et al. Variables affecting human vertebral body marrow yields. Transplant Proc 1997; 29(4): 1959. (back)
- Kong SS, Kenyon NS, Brendel M, Tzakis AG, Miller J, Ricordi C. Effect of preservation conditions on human vertebral body marrow. Transplant Proc 1995; 27(6): 3415. (back)
- Bottino R, Linetsky E, Selvaggi G, Kong SS, Qian T, Ricordi C. Automation of human vertebral body bone marrow isolation. Ann N Y Acad Sci 1995; 770: 364-365. (back)
- Bottino R, Linetsky E, Selvaggi G, Kong SS, Qian T, Ricordi C. Human vertebral body bone marrow harvest: comparison between manual and automated methods. Transplant Proc 1995; 27(6): 3340. (back)
- Kenyon NS, Xu XM, Knapp J, Selvaggi GS, Bottino R, Kong SS, et al. Automated processing of human vertebral body bone marrow yields preparations with stem cell content similar to that obtained with traditional manual preparation. Transplant Proc 1995; 27(6): 3418. (back)
- Kong SS, Selvaggi G, Kenyon N, Bottino R, Linetsky E, Ricordi C. A simple method for depletion of bone fragments from human vertebral body marrow. Transplant Proc 1995; 27(6): 3417. (back)
- Abrahams C, Catchatourian R. Bone fragment emboli in the lungs of patients undergoing bone marrow transplantation. Am J Clin Pathol 1983; 79(3): 360-363. (back)
- FACT-JACIE. International Standards for Cellular Therapy Product Collection, Processing, and Administration. The Foundation for the Accreditation of Cellular Therapy and the Joint Accreditation Committee International Standards; 2012. (back)
- AABB. Standards for Hematopoietic Progenitor Cell and Cellular Product Services. American Association of Blood Banks; 2013. (back)
To cite this article
Human Vertebral Body Marrow Processing: Standard Operating Procedure from the cGMP Human Cell Processing Facility of the Cell Transplant Center and Diabetes Research Institute at the University of Miami
CellR4 2013; 1 (3): e557
Publication History
Published online: 22 Nov 2013

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.