CellR4 2013; 1 (2): e377
Mesenchymal Stem Cells And Solid Organ Transplantation
Topic: Regenerative Surgery
Category: Reviews
Abstract
Mesenchymal stem cells are multipotent stem cells derived from various sources. This review describes their immunomodulatory effect on T-cells, B-cells, NK cells and dendritic cells and interactions with T-regulatory (CD4+/25high Foxp3 +) cells, the last being one of the most important mode of actions. These cells are “super-suppressive” cells which act in “cell-cell contact” or through release of cytokines and growth factors. The mechanism of immune-modulation is species specific. MSC has contributed significantly towards the evolution of “cell therapy” in transplantation immunobiology. Sparse data on clinical use of MSC in organ transplantation are available. Our experience is consistent with the prevailing notion that “cell therapy” with MSC in the lead will carry the torch of therapeutic avenues yet not explored.
Abbreviations: AD-MSC: adipose tissue derived mesenchymal stem cells; APC: antigen presenting cell; BM: bone marrow; CNI: calcineurin inhibitor; DC: dendritic cells; GVHD: graft-versus-host disease; HLA: histocompatibility locus antigen; HGF: hepatocyte growth factor; IL: interleukin; IDO: indoleamine 2,3-dioxygenase; MLR: mixed lymphocyte reaction; MSC: mesenchymal stem cells; PGE2: prostaglandin E2; SC: stem cells; TGF: transforming growth factor; TH: T-helper; T-regs: T-regulatory cells.
INTRODUCTION
Mesenchymal stem cells (MSC) are multipotent stem cells (SC) derived from various sources. They were first isolated in 1974 from bone marrow (BM) by Friedenstein and Petrokova 1. In undifferentiated state, they appear fibroblastoid and have small cell body with few long and thin cell processes. There are no specific markers to identify them; however, they are negative for hematopoietic cell markers like CD34/45/HLA-DR and express CD90/73/105 on their surface 2 , 3 , 4. They have the plasticity to differentiate in to mesenchymal and non-mesenchymal cell types alike, both in vitro and in vivo 5 , 6 , 7. Human adipose tissue derived MSCs (AD-MSC) are morphologically similar to their counterparts in BM; however, their proliferation and differentiation capacity is higher 8. The International Society for Cellular Therapy has recommended the following minimum criteria for defining multi-potent human MSCs 9 , 10 (i) adherence to plastic under standard culture conditions; (ii) positive for expression of CD105, CD73 and CD90 and negative for expression of the haematopoietic cell surface markers CD34, CD45, CD11a, CD19 or CD79a, CD14 or CD11b and histocompatibility locus antigen (HLA)-DR; and (iii) under a specific stimulus, differentiation into osteocytes, adipocytes and chondrocytes in vitro. One of the most intriguing features of MSCs is that they escape immune recognition and can inhibit immune responses 11. Because of their unique regenerative potential, MSCs exhibit potential for use in tissue regeneration and repair for conditions such as cardiac anomalies or injury, bone disorders and metabolic diseases.
IMMUNOMODULATORY FUNCTIONS OF MSCS
T cell proliferation and function
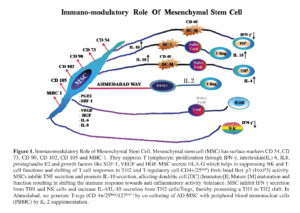
MSCs can suppress the T lymphocyte proliferation induced by alloantigens, mitogens and anti-CD3 and anti-CD28 antibodies in vitro, in humans, baboons and mice 12 , 13 , 14 15 , 16 , 17 , 18. MSCs have a similar effect on memory and naive T cells as well as CD4+ and CD8+ T cells and this suppressive effect does not require major histocompatibility complex (MHC) restriction 19 , 20 , 21. Cell inhibition is believed to be due to soluble/growth factors like IFN-γ, interleukin [IL]-1β, Transforming growth factor (TGF)-β1 and hepatocyte growth factor (HGF) in humans 22. Their immunomodulatory activity is believed to be through these growth factors and indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2) 23 , 24 , 25. The secretion of human leucocyte antigen-G5 [HLA-G5] by MSCs is reported to be essential for their following effects: suppression of T-cell and NK cell function, shift of the allogeneic T-cell response to a T-helper type 2 (Th2) cytokine profile and induction of CD4+CD25high forkhead box P3 (FoxP3+) regulatory T cells (Tregs) 26 , 27.
B cell proliferation and function
The major mechanism of B cell suppression by MSCs is attributed partly to the physical contact between MSCs and B cells and in part to the soluble factors released by MSCs; this leads to the blocking of B cell proliferation in the G0/G1 phase of the cell cycle with no apoptosis 28 , 29 , 30. MSCs inhibit the proliferation of B cells activated with anti-immunoglobulin (Ig) antibodies, anti-CD40L antibody and cytokines (IL-2 and IL-4) 31.
Modulation of natural killer (NK) cells
Soluble factors such as TGF-β1 and PGE2 are believed to play a role in the MSC-mediated suppression of NK cell proliferation 32.
Interaction between MSCs and dendritic cells (DCs)
MSCs impair the differentiation of monocytes or CD34+ HSCs into DCs by inhibiting the response of the former to maturation signals, reducing the expression of co-stimulatory molecules and hampering the ability of the former to stimulate naive T cell proliferation and IL-12 secretion 33 , 34 , 35. In addition, this inhibitory effect might be mediated via soluble factors and may be dose-dependent 36. MSCs isolated from human adipose tissue are more potent immunomodulators for the differentiation of human DCs than MSCs derived from the BM 37.
Induction of T-regulatory cells (Tregs)
MSCs may also modulate immune responses via the induction of Tregs. MSC can induce the generation of CD4+CD25+ cells displaying a regulatory phenotype (FoxP3+) in mitogen-stimulated cultures of peripheral blood mononuclear cells although the functional properties of these cells have not yet been elucidated 38 , 39. Ge W et al 40 reported that MSCs could induce kidney allograft tolerance by inducing the generation of CD4+CD25+FoxP3+ Tregs in vivo. Additionally, MSCs have been reported to induce the formation of CD8+ Tregs that are responsible for the inhibition of allogeneic lymphocyte proliferation 41. The induction of Tregs by MSCs involves not only direct contact between MSCs and CD4+ cells, but also the secretion of soluble factors such as PGE2 and TGF-β1 42. Human gingiva-derived MSCs can induce IL-10, IDO, inducible NO synthase and cyclooxygenase 2 thereby serving as immunomodulatory components in the treatment of experimental inflammatory diseases 43. A study has shown that the immunosuppressive effect of MSCs is mediated by the secretion of galectin-3, a protein known to modulate T cell proliferation, gene expression, cell adhesion and migration 44. MSCs have also shown to prevent autoimmune B cell destruction and subsequent diabetes in NOD mice by inducing Tregs 45. The effect of MSCs in the treatment of autoimmune diseases may be through the induction of de novo generation of antigen-specific CD4+CD25+FoxP3+ Tregs 46 , 47. However, a recent study reported that MSCs could sustain or suppress T-cell proliferation depending on their concentration, and a low MSC/T-cell ratio might support T cell proliferation 48. In a recent study by Siod et al they found that MSCs could stimulate the activation and proliferation of resting T-cells and generate Tregs 49. These data suggest that the culture conditions play an important role in the clinical application of MSCs 50.
MSC and Transplantation Tolerance
Immunomodulatory role of MSCs in vitro and in vivo in experimental models has led to the evolution of “cell therapy” as a new branch for exploring therapeutic applicability in auto-immune disorders and allo-immune conditions which are otherwise not amenable to other therapeutic modalities.
In pathological conditions, MSCs migrate preferentially into lymphoid organs, allografts, injured and/or inflammatory tissue sites after systemic transfusion, where they interact with the activated immune cells and modulate their function 51 , 52. Bartholomew et al 53 first described the in vivo immunomodulatory properties of MSCs in a baboon model of skin transplantation. The therapeutic potential of MSCs in immunomodulation is being explored currently in several Phase I, II and III clinical trials 54 many of which have recently been completed or are under way, as reported in the clinical trials website of the United States sponsored by the National Institutes of Health [http://clinicaltrials.gov]. Because of their immunosuppressive properties, MSCs are believed to play a role in the maintenance of peripheral tolerance and the induction of transplantation tolerance, and they are considered potential candidates in cellular therapy for graft-versus-host disease (GVHD) and autoimmune diseases and in protecting solid-organ grafts from being rejected 55. MSCs derived from umbilical cord, BM and occasionally adipose tissue are being tried or considered for clinical trials 56. MSCs obtained from HLA-identical sibling donors, haplo-identical donors and third-party HLA-mismatched donors infused in 55 patients with steroid-refractory acute GVHD were shown to elicit a response in more than half the patients; the study showed that MSCs exerted their therapeutic effect in the case of both HLA-matched and HLA-unmatched donors. However, for GVHD, the use of MSCs is a double-edged sword, because the prevention of GVHD was associated with a high incidence of leukaemia relapse, which is the result of the non-specific immunosuppressive effect of MSCs on graft-versus-leukaemia 57 , 58. Liang et al 59 reported that allogeneic MSC transplantation in patients with refractory SLE resulted in the amelioration of disease activity, improvement in the levels of serological markers and stabilization of renal function without the occurrence of serious adverse events. For solid organ transplantation, the beneficial effect of MSC-based immunosuppressive therapy is debatable. The application of calcineurin inhibitors (CNIs) would abrogate the immunosuppressive effect of MSC therapy. In addition, CNIs cause renal failure, hypertension and hyperglycaemia, and increase the risk of malignancy; therefore, efforts have been made to minimize the use of CNI in organ transplantation protocols.
Embryonic, hematopoietic and mesenchymal stem cells have been successfully employed for tolerance induction in a variety of rodent and large-animal studies 60 , 61 , 62. MSCs are not only able to evade the immune system, but they can also suppress immune responses directed against third-party cells, even inducing tolerance toward other tissues of the same origin when transplanted following intravenous infusion of MSCs 63. This and other studies have further demonstrated that MSCs inhibit T-cell activation ex vivo 64 , 65 , 66 , 67. A case report by Le Blanc et al 68 suggested that systemic infusion of haplo-identical MSCs suppressed a grade IV GVHD in a 9-year-old child who had received a BM transplant (BMT). Ringden et al 69 reported the administration of MSC with median dose of 1.0 ×106/kg to eight patients with steroid-refractory grades III-IV acute GVHD and one with extensive chronic GVHD 70. No acute side-effects occurred after the MSC infusions. Six patients were treated once and three patients twice. Two patients received MSC from HLA-identical siblings, six from haplo-identical family donors and four from unrelated mismatched donors. Acute GVHD disappeared completely in six of eight patients. One of these developed cytomegalovirus gastroenteritis. Complete resolution was seen in gut (6), liver (1) and skin (1). Two died soon after MSC treatment with no obvious response. One of them had MSC donor DNA in the colon and a lymph node. Five patients were reported alive between 2 months and 3 years after the transplantation. Their survival rate was significantly better than that of 16 patients with steroid-resistant biopsy-proven gastrointestinal GVHD, not treated with MSC during the same period (p = 0.03). One patient treated for extensive chronic GVHD showed a transient response in the liver, but not in the skin and he died of Epstein-Barr virus lymphoma. This study showed that MSC have very promising treatment for severe steroid-resistant acute GVHD. The underlying mechanism for this tolerizing phenomenon, including the involved target cells, is not yet known.
Tolerance induction in the periphery is believed to be critical for the prevention of autoimmunity and maintenance of immune homeostasis. Central tolerance has been classically ascribed to clonal deletion of self-reactive T-cells in the thymus upon interaction with self-antigens. However, central tolerance is incomplete because not all self-antigens gain access to the thymus, and several self-reactive lymphocytes escape central deletion. In the past several years there has been growing evidence supporting this notion, revealing subpopulations of cells representing different arms of the immune system, as potential regulators of the immune system. These specific groups include T-cell subtypes (such as CD4+CD25+ T cells), as well as a unique fraction of DCs described as semimmature DCs, all of whom were shown to possess immune-modulating properties. Therefore, “sentinels” in the periphery of the body are essential to maintain tolerance as well as immunity. These tolerogenic effectors, while constitutively active in autoimmunity prevention, may play a pivotal role in maternal-fetal non-rejection, as well as in immune evasion of tumors and metastases.
MSC evade allo-rejection
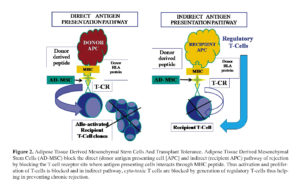
The major limit to solid organ graft survival is T-cell recognition by the recipient of alloantigen (dominated by, but not confined to MHC/HLA antigens) 71. There are two mechanisms mediating this powerful rejection response; “direct” recognition, involving recognition by recipient CD8+ or CD4+ T-cells of donor MHC class I and class II molecules; and “indirect” mechanisms involving recognition of peptides from the allogeneic tissue 72. Recipient antigen presenting cells (APC) such as DC process alloantigen into peptides and present these to naive T cells on self-MHC molecules 73. However there are notable exceptions to these allorejection processes; the fetal allograft evades rejection by the mother through a complex series of actions, similarly tissue which has limited lymphatic drainage is less prone to allorejection 74 , 75. Interestingly tumor cells, whilst not allogeneic, are in many cases both “altered-self” and immunogenic but often actively modulate immune responsiveness to evade immune surveillance 76. Thus mechanisms of tumor evasion of the immune system may provide insight into how allogeneic MSC are tolerated by the mismatched host.
There is supporting evidence for the use of allogeneic MSC from both in vitro and in vivo studies that show MSC avoid normal allo-responses. A small number of in- vivo studies suggest that MSC play a role in enabling alloantigen tolerance. Koc et al 77 showed no evidence of alloreactive T cells and no incidence of GVHD when allogeneic MSC were infused into patients with Hurler’s syndrome or metachromatic leukodystrophy. In a previous study by the same group 78, autologous culture-expanded MSC were infused to breast cancer patients to investigate whether MSC would enhance the engraftment of peripheral blood stem cells after myeloablative therapy. Results showed rapid hematopoietic recovery and no signs of toxicity from MSC infusion. Horwitz et al 79 reported that donor MSC contributed to bone remodelling after allogeneic stem cell transplantation (SCT) in 3 children with osteogenesis imperfecta, a rare genetic disorder of type I collagen. This is supported by data from Bartholomew et al 80 who showed that in-vivo administration of allogeneic MSC prolonged 3rd party skin graft survival in animal models. Furthermore, Saito et al 81 demonstrated that MSC undergoing differentiation to a cardiac phenotype were tolerated in a xenogeneic environment, retaining their ability to be recruited to the injured myocardium. More recent work by Aggarwal and Pittenger 82 supported the feasibility of MSC-transplantation showing that MSC altered the phenotypes of specific immune cell subtypes thereby creating a tolerogenic environment. These reports suggest that transplantation of MSC could be beneficial in patients with various disorders requiring tissue regeneration, and provide evidence supporting the tolerance of allogeneic MSC by recipients.
Data supporting the contention that MSC avoid allogeneic responses has also come from a large body of in vitro experiments, usually involving co-culture or mixed lymphocyte reactions (MLR). Evidence from these studies indicates that the use of mismatched MSC does not provoke a proliferative T cell response in allogeneic MLR, thus suggesting an immunosuppressive role for MSC 83 , 84 , 85 , 86 , 87 , 88. Le Blanc et al 89 showed that MSC failed to elicit proliferation of allogeneic lymphocytes. Additionally, they demonstrated that MSC remained immunosuppressive even after IFN-γ stimulation. Krampera et al 90 confirmed these findings, they showed that murine MSC lack MHC class II and inhibited T cell proliferation. Tse et al 91 also showed that human MSC fail to elicit allogeneic T cell response in MLR even when MHC class II was upregulated. Consistent with these studies, Bartholomew et al 92 showed that allogeneic baboon MSC suppressed the proliferative activity of lymphocytes in vitro and prolonged graft survival. These findings support the view that MSC can be transplanted between MHC-incompatible individuals. Although these data show that successful use of allogeneic MSC in regenerative therapy is possible, such approaches are unlikely to be broadly acceptable until it is understood why MSC are not rejected. This question has been the subject of intense recent study and three candidate mechanisms are emerging. MSC appear to evade allogeneic rejection by (a) being hypoimmunogenic; (b) modulating T cell phenotype and (c) creating an immunosuppressive local milieu. These mechanisms are inter-related and will involve cell contact dependent and independent interactions. The challenge facing the field is to unravel the contribution of these diverse interactions.
MSC are hypoimmunogenic
There is controversy surrounding the cell surface expression of MHC alloantigens by MSC. Although conflicting evidence exists, most studies describe human MSC as MHC class I positive and MHC class II negative. The data conflicting with these findings may represent different stem cell lineages or be the result of the recently described process of cell-cell transfer 93 , 94 , 95. The expression of MHC class I by MSC is important because expression protects MSC from certain NK cell mechanisms of deletion. HLA-G is an MHC-like protein that is known to protect the fetal allograft against NK mediated rejection 96 , 97. This protein has been shown to bind to the two major inhibitory NK receptors, KIR1 and KIR2, and to inhibit NK killing 98 , 99 , 100. However no studies of HLA-G expression by MSC have been reported to date.
As MHC class II proteins are potent alloantigens, the expression by MSC is another important factor. Again there is some controversy over expression, which may be explained by the diversity of models described above. However there are widespread observations that under non-inflammatory conditions, human MSC are MHC-II negative, supporting a role for MSC as having reduced immunogenicity through the control of alloantigen expression 101 , 102 , 103. The absence of MHC class II gives MSC the potential to escape recognition by alloreactive CD4+ T cells. In addition to being MHC II negative, MSC do not appear to express the co-stimulatory molecules CD40, CD40L, CD80 or CD86 required for effector T cell induction 104 , 105. The absence of co-stimulatory molecules is a significant observation. It implies that any residual engagement of the T cell receptor on Th cells would result in anergy and contribute to tolerance rather than allogeneic responses. Although this is a comforting scenario, based largely on in vitro studies, it cannot fully explain the evasion of alloreactivity demonstrated by MSC. Experiments involving allogeneic co-cultures or MLR have demonstrated that both cell-cell contact and action by soluble factors contribute to the immunomodulatory function of MSC 106 , 107 , 108 , 109. Thus it is likely that evasion of alloreactivity is a result of both MSC hypoimmunogenicity, modulation of T cell immune induction and the creation of a suppressive milieu around MSC. Although the mechanisms governing the suppressive effect are not fully understood, several studies have given indicators to the processes involved.
MSC interfere with DC maturation and function
DC are the most influential APC, playing a key role in directing cellular and humoral immune responses against self and non-self antigens 110. DC contribute to the establishment of tolerance, especially in the periphery 111. Immature DC are not fully differentiated to carry out their known role as inducers of immunity 112. Despite this, immature DC circulate through tissues and the lymph system, capturing self and non-self antigens 113. Immature DC that are loaded with antigen can silence T cells by deletion or by expanding Treg populations 114 , 115. It has long been believed that this process contributes to graft survival during transplantation 116. The capacity of DC to induce peripheral tolerance is a potential mechanism by which MSC could manipulate immunity in order to escape T cell recognition. Thus MSC could prevent normal allogeneic responses either through modulation of DC function or by direct effects on T cells. Indications from different studies encourage this hypothesis. Zhang et al provide evidence that MSC interfere with DC maturation26. Co-culture experiments showed that MSC down-regulate CD1a, CD40, CD80, CD86, and HLA-DR expression during DC maturation. This is also shown by Beyth et al 117 who suggest that human MSC converted APC into an inhibitory or suppressor phenotype via cell-to-cell contact, thus locking DC into a semi-mature state and thereby inducing peripheral tolerance. Their findings also show reduced IFN-γ, IL-12 and TNF-α in human MSC/monocyte co-culture. Similarly Jiang et al 118 reported that MSC maintain DC in an immature state and show that MSC inhibit up regulation of IL-12p70. These results suggest that MSC mediate allogeneic tolerance by directing APC towards a suppressor or inhibitory phenotype that results in an attenuated or regulatory T cell response.
MSC modulate CD4+ T cell responses
Evidence has emerged that MSC interact directly with T cells to suppress alloreativity. Krampera et al 119 showed that MSC impair T cell contact with APC in a non-cognate but transient fashion. This supported work from Bartholomew et al 120 showing that the addition of IL-2 to MLR/MSC co-cultures reduced MSC suppression and restored T cell proliferation. Taken together, these results strongly support a role for either a direct (T cell phenotype) or indirect (DC phenotype) mechanism of immune modulation directed by MSC. MSC modulation of CD4+ T cell responses is more extensive than the straightforward effect described above. The regular process of antigen specific CD4+ T cell induction requires antigen capture and processing by DC (or other amenable cells), followed by a process of maturation and trafficking to local lymph nodes 121 , 122 , 123 , 124. There is evidence that MSC prevent normal allogeneic responses by directing CD4+ T cells to a suppressive or counter-regulatory phenotype 125 , 126. Di Nicola et al 127 showed that MSC strongly suppressed CD4+ (and CD8+) T cells in MLR findings supported by Tse et al 128, who showed that MSC suppress the proliferation of T-cell subsets. Studies of T cell differentiation have shown that in the presence of human MSC, Th1 cell secretion of IFN-γ dropped by 50% compared to cultures without MSC. Conversely, effector T cells undergoing Th2 differentiation when co-cultured with human MSC showed a significant increase in IL-4 production compared to controls 129. These findings suggest that MSC exert a counter regulatory, anti-inflammatory role by directing cytokine-mediated immunity 130. A strategy of regulation and deletion of specific T cells is an effective control against unwanted immune responsiveness especially after transplantion85. Consequently, enormous interest has focused on the possibility of Tregs as markers for T cell tolerance during transplantation. Treg can act directly on other T cells or indirectly through APC80. Aggarwal et al demonstrated that CD4+ CD25+ T reg populations increased significantly in MLR when MSC were present compared to controls 131. However, data exists showing that human MSC-mediated inhibition is not suppressed by removing Tregs from co-cultures 132 , 133. Nevertheless a role for these cells can not be excluded, it is possible that an incomplete replication of the suppressive microenvironment in vitro or indeed the diversity of Treg populations mean that these studies do not fully explore the potential role of suppressive or regulatory T cells in promoting MSC tolerance.
MSC influence control over cell division cycle pathways in cells of immunological relevance. Glennie et al 134 have shown that T cells stimulated in co-cultures with MSC exhibit an extensive inhibition of cyclin D2 and upregulation of the cyclin dependent kinase inhibitor p27kip1. As T cell inhibition could not be reversed, these cells were not interpreted as anergic in the classical sense. The authors suggest that MSC are most likely inducing the alternative condition of divisional arrest anergy in T cells, an occurrence usually associated with CTLA-4 signalling 135. In addition, removal of MSC from the system only restored IFN-γ production but not T cell proliferation 136. This suggests that MSC induce a condition similar to split anergy 137 or split tolerance 138 , 139. The key point is that this work demonstrates that MSC exert veto effects on T cells and it is significant in demonstrating that the mechanisms inducing MSC tolerance are not confined to patterns of cytokine secretion but extend to direct modulation of T cell division.
MSC modulate CD8+ T cell and NK cell activity
The impact of MSC on CD8+ CTL and NK cells has also been addressed. CTL can lyse allogeneic cells after recognition of cognate alloantigen, by the release of cytotoxic effectors such as perforins, serine esterases, IFN-γ and TNF-α 140 whereas NK cells do not require antigen processing 141. Consequently both effector cells can operate in tandem, with NK cells providing a first line defense killing target cells that escape CTL recognition or show inadequate expression of self-MHC 142. There is evidence that MSC inhibit the formation of CTL and appear to evade NK cell targeting mechanisms. Djouad et al14 showed that CD8+ cells are suppressed by MSC in MLR. Rasmusson 143 supported these findings and further showed that NK cells in co-culture did not recognize MSC although lytic capability was still present. This effect appeared to be mediated by soluble factors 144 , 145. Thus MSC interact and suppress cell-mediated immune responses directly and through soluble factors. The targets for this suppression are DC, CD4+ Th, CD8+ CTL and NK cells; in effect MSC silence each aspect of the cellular rejection process.
MSC secrete soluble factors to create an immunosuppressive milieu
The characterization of cytokines produced by MSC is still provisional and is hindered by the lack of standardization in isolation and culture conditions, which have given rise to multiple findings and interpretations. It is evident that MSC do not constitutively express IL-2, IL-3, IL-4 and IL-5 146 , 147. However, some reports show that MSC do constitutively express mRNA for cytokines such as IL-6, -7, -8, -11, -12, -14, -15, -27, leukaemia inhibitory factor, macrophage colony-stimulating factor, and stem cell factor 148 , 149. Some of these cytokines provide critical cell-cell interactions and promote HSC differentiation, however caution should be exercised before over interpreting these findings. Protein secretion does not always mirror mRNA levels and most workers in the field would adopt a more conservative profile of cytokine and growth factor production by MSC.
Despite these caveats, certain MSC secreted products such as HGF are likely to contribute to creating a local immunosuppressive environment. HGF induces mitogenic and anti-apoptotic activity in different systems 150 , 151 , 152 and has a well-characterized role in wound repair 153 , 154 , 155, effects that are consistent with a role for MSC in regenerative medicine. Although some groups do not detect HGF in MSC co-cultures 156 more reports suggest that HGF is constitutively expressed by MSC 157 , 158 , 159 , 160. Indications that MSC produce HGF 161 , 162 , 163 , 164 encourage a role for these cells in tissue repair 165. Studies by Chunmeng et al 166 demonstrated that rat dermal derived “multipotent” cells secrete HGF and promote wound healing. Interestingly, Azuma et al 167 showed that HGF treatment prevents chronic allograft nephropathy in rats. Taken together these results suggest that HGF may contribute to the ability of MSC to avoid allo-rejection. IL-10 has a well-documented role in T cell regulation and in the promotion of a “regulatory” or suppressor phenotype. IL-10 is likely to be suppressing potential allo-responsiveness because it is a recognized growth factor for regulatory T cells 168. IL-10 can antagonize IL-12 during induction of inflammatory immune responses 169 , 170 , 171 , 172 , 173 , 174. MSC constitutively express the eicosanoid PGE-2 175. This may be upregulated in co-culture 176 , 177 or down-regulated on differentiation 178. PGE-2 influences numerous immune functions including suppression of B cell activation 179 and induction of regulatory T cells 180. MSC control surface marker expression to exhibit a hypoimmunogenic or tolerogenic phenotype. MSC can also modulate T cell induction directly or via DC and secrete a battery of immunosuppressive factors.
MSC avoidance of alloreactivity shows parallels to tumor evasion
Escape from immune surveillance is believed to be a primary feature of malignant disease in humans. The immune effector response is sub-optimal because tumors develop multifactorial strategies to escape immune deletion 181 , 182. These strategies may provide clues to how MSC promote tolerogenic mechanisms during allogeneic engraftment. Modulation of tumor antigen expression, particularly MHC class I and II is a particularly common component of tumor immune evasion 183. This is often accompanied by poor or non-expression of co-stimulatory molecules, which not only limits clonal expansion of tumor-specific CD4+ T cells, but also hinders the production of cytokines, and the development of CTL 184 , 185 , 186. Similarly MSC show no expression of co-stimulatory molecules 187 , 188. In addition to reduced immunogenicity, tumor cells can directly modulate DC and T cell function. Studies from patients with hepatocellular carcinoma showed that neoplasia induced a defect of DC maturation 189. This parallels findings by Beyth et al 190 suggesting that human MSCs interfere with normal APC maturation, thereby indirectly influencing T-cell activation. Freshly isolated tumor-infiltrating T cells are usually inactive against autologous cancer cells but can be reactivated in-vitro by the addition of IL-2 191. Studies of MSC by Le Blanc et al 192 showed striking parallels to this form of suppression. They suggest that MSC act by preventing expression of CD25 (IL-2 receptor) thereby limiting T cell activation 193. Other work has shown that exogenous IL-2 addition to co-cultures containing MSC reversed the suppressive effect 194 , 195. Similarly, antigen-specific CD4+ CD25+ regulatory T cells also suppress tumor-specific CD8 T cell cytotoxicity although this mechanism relies on TGF-β secretion by regulatory cells 196 , 197.
Tumors can suppress CD4+ T cell activity and CTL tumor lysis directly through secretion of immunosuppressive factors including TGF-β1 but also PGE-2, and IL-10. Van der Pouw Kraan et al 198 showed that tumor-derived prostaglandins increased the production of inhibitory cytokines such as IL-10, while suppressing IL-12, which is necessary for effective host-cell-mediated anti-tumor immune response 199 , 200. Likewise, TGF-β production has been reported from a number of tumors, contributing to immune evasion. Intriguingly in this context it also inhibits CTL differentiation 201. Although there is little evidence that MSC secrete TGF-β1, the bone marrow is rich in this cytokine, suggesting that MSC reside in a compartment with immunosuppressive qualities.
Although there are striking parallels between MSC and some tumor cells, these cells are not directly related. There are distinct differences between the populations. The fundamental difference between the cell types resides in the control of cell division and apoptosis, which are tightly regulated in MSC but dys-regulated in transformed cells 202. Some tumors exploit FasL [CD95L] expression to facilitate immune escape 203 , 204 , 205. It appears that MSC retain certain aspects of the fetal allograft that promote tolerance, some of these mechanisms may be reactivated in neoplasia, the key difference being that MSC perform these functions in an ordered and controlled way whereas tumor cells do so in a manner that by definition has escaped normal controls on apoptosis or cell division 206 , 207.
MSC and solid organ transplantation-
Time and dose Relationship
Casiraghi et al 208 studied the tolerogenic effect of MSC in a semiallogeneic heart transplant mouse model. They found out that pre-organ transplant infusion of MSC in 1 or 2 doses [on day-7 and day-1] induced a profound T cell hyporesponsiveness and prolonged B6C3 cardiac allograft survival. The pro-tolerogenic effect was abrogated when donor-derived MSC were injected together with B6C3 HSC, suggesting that HSC negatively impact MSC immunomodulatory properties. Both the induction [pretransplant] and the maintenance phase [>100 days posttransplant] of donor-derived MSC-induced tolerance were associated with CD4+CD25+Foxp3+ Treg expansion and impaired anti-donor Th1 activity. MSC-induced Tregs were donor-specific since adoptive transfer of splenocytes from tolerant mice prevented the rejection of fully MHC-mismatched donor-specific secondary allografts but not of third-party grafts. In addition, infusion of recipient-derived B6 MSC tolerized a semiallogeneic B6C3 cardiac allograft, but not a fully MHC-mismatched BALB/c graft, and expanded Treg. A double intravenous pretransplant infusion of recipient-derived MSC had the same tolerogenic effect as the combined intraportal and intravenous MSC infusions, which makes the tolerogenic protocol applicable in a clinical setting. In contrast, single MSC infusion given either peritransplant or 1 day after transplant were less effective. Altogether these findings indicate that MSC immunomodulatory properties require HSC removal, partial sharing of MHC Ags between the donor and the recipient and pretransplant infusion, and are associated with expansion of donor-specific Treg. In another study by the same group 209, they found that although MSCs administered post-transplant promoted neutrophil infiltration and complement deposition, infusion of MSCs pretransplant induced significant allograft survival through a Treg dependent mechanism. The key observation of this study was that MSCs infused pretransplant localize in the lymphoid organs whereas MSC administered posttransplant are recruited to the graft [syngeneic or allogeneic]. Overall, MSCs can exert protective effects in ischemic reperfusion injuries through anti-inflammatory and paracrine factors and this likely plays an important part in MSC enhancement of allograft survival.
There are rare reports on clinical application of MSC in solid organ transplantation. The largest series of clinical use of MSC in solid organ transplantation is reported by our group. We have been using combined donor derived HSC and adipose tissue derived MSC infusion in living related renal transplantation 210. In a cohort of 916 patients we infused CD34+ cells, 1.58 ± 1.62 x 106/kgBW, CD90+ cells, 3.54 ± 2.57 x 103/kgBW and CD73+ cells, 1.04 ± 1.25 x 103/kgBW pretransplant under nonmyeloablative conditioning. We compared this group with 310 matched controls. We have observed significantly better graft and patient survival in stem cell group vs. controls with additional benefits of minimization of immunosuppression. We have an interesting observation [unpublished data] that adipose tissue derived MSC help in recruitment and generation of Tregs thus promoting tolerogenic effects of MSC. We have also generated T-regs (CD4+/25high/127low/-) in vitro from donor AD-MSC and recipient peripheral blood mononuclear cells and these T-regs are infused in thymus of renal allograft recipients after kidney transplantation. This has helped in further fortifying graft function by alleviating rejection episodes and minimizing immunosuppression requirement (unpublished data).
In a pilot study reported by Andy Peng Xiang et al 211, donor-derived BM-MSCs combined with a 50% standard dose of tacrolimus were administered to 6 LDRT recipients. Six other patients who received a standard dose of tacrolimus were enrolled as a control. Over a 1 year follow-up, none of the MSC recipients experienced immediate or long-term toxic side effects associated with MSC infusion. The tacrolimus dose in the MSC group was significantly reduced compared with the control group. One acute rejection occurred only in the control group. All patients survived with stable renal function at month 12 and no chimerism was detectable at month 3. Patients in the MSC group showed significantly higher B-cell levels than the control group at 3 month post-transplant. In another study by Tan et al 212, 104 patients were inoculated with autologous BM-MSC (1-2 x106/kg) at kidney reperfusion and two weeks later. Fifty-three patients received standard-dose and 52 patients received low-dose CNIs (80% of standard); 51 patients in the control group received anti–IL-2 receptor antibody plus standard-dose CNIs. After 6 months, 4 of 53 patients in the autologous MSC plus standard-dose CNI group and 4 of 52 patients in the low-dose group had steroid responsive biopsy-proven rejections compared with 11 of 51 controls who had steroid-resistant rejections. Renal function recovery was better in patients who received MSC vs. controls. Also, during the 1-year follow-up, combined analysis of MSC-treated groups revealed significantly decreased risk of opportunistic infections than the control group. In a study by Marlies E.J. Reinders et al 213, they administered two intravenous infusions (1 million cells per kilogram) of autologous BM-MSCs in 6 LDRT recipients. Protocol renal biopsy at 4 weeks or 6 months showed signs of rejection and/or an increase in interstitial fibrosis/tubular atrophy (IF/TA) in 2 patients. Although maintenance immunosuppression remained unaltered, there was a resolution of tubulitis without IF/TA in both patients. Additionally, three patients developed an opportunistic viral infection, and five of the six patients displayed a donor-specific downregulation of the peripheral blood mononuclear cell proliferation assay, not reported in patients without MSC treatment 214.
It is to be noted that all research other than Trivedi et al, has been carried out on BM derived MSC and all trials have been performed after solid organ transplantation. Hence the results are not as encouraging as noted by our group.
To conclude, enough bench work on MSC is available however larger multi-center clinical trials of MSC in solid organ transplantation are required to confirm their tolerogenic properties.
Conflict of Interests
The Authors declare that they have no conflict of interests.
No financial support was received from any source for the work mentioned/for manuscript.
- Friedenstein AJP, Petrokova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphology 1966; 16: 381-390. (back)
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284(5411): 143-147. (back)
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418 (6893): 41-49. (back)
- Reyes M, Lund T, Lenvik T, et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood 2001; 98: 2615-2625. (back)
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284(5411): 143-147. (back)
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002; 418 (6893): 41-49. (back)
- Reyes M, Lund T, Lenvik T, et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood 2001; 98: 2615-2625. (back)
- Reyes M, Verfaillie CM. Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann N Y Acad Sci 2001; 938: 231-235. (back)
- Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy 2005; 7: 393-395. (back)
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315-317. (back)
- Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res 2006; 312: 2169-2179. (back)
- Di Nicola M, Carlo-Stella C, Magni M et al. Human bone marrow stromal cells suppress T lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838-3843. (back)
- Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003; 57: 11-20. (back)
- Potian JA, Aviv H, Ponzio NM, et al. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol 2003; 171: 3426-3434. (back)
- Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003; 75: 389-397. (back)
- Bartholomew A, Sturgeon C, Siatskas M et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30:42-48. (back)
- Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003; 102: 3837-3844. (back)
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003; 101: 3722-3729. (back)
- Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003; 57: 11-20. (back)
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003; 101: 3722-3729. (back)
- Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005; 105: 2821-2827. (back)
- Di Nicola M, Carlo-Stella C, Magni M et al. Human bone marrow stromal cells suppress T lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838-3843. (back)
- Di Nicola M, Carlo-Stella C, Magni M et al. Human bone marrow stromal cells suppress T lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838-3843. (back)
- Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase mediated tryptophan degradation. Blood 2004; 103: 4619-4621. (back)
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815-1822. (back)
- Carosella ED, Moreau P, Le Maoult J, et al. HLA-G molecules: from maternal–fetal tolerance to tissue acceptance. Adv Immunol 2003; 81: 199-252. (back)
- Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008; 26: 212-222. (back)
- Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005; 105: 2821-2827. (back)
- Augello A, Tasso R, Negrini SM, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol 2005; 35: 1482-1490. (back)
- 22 (back)
- Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006; 107:367-372. (back)
- Sotiropoulou PA, Perez SA, Gritzapis AD, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 2006; 24: 74-85. (back)
- Jiang XX, Zhang Y, Liu B et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005; 105: 4120-4126. (back)
- Nauta AJ, Kruisselbrink AB, Lurvink E, et al. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol 2006; 177: 2080-2087. (back)
- Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev 2004; 13: 263-271. (back)
- Nauta AJ, Kruisselbrink AB, Lurvink E, et al. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol 2006; 177: 2080-2087. (back)
- Ivanova-Todorova E, Bochev I, Mourdjeva M, et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett 2009; 126: 37-42. (back)
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815-1822. (back)
- Maccario R, Podesta M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 2005; 90: 516-525. (back)
- Ge W, Jiang J, Arp J, et al. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation 2010; 90: 1312-1320. (back)
- Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003; 102: 3837-3844. (back)
- English K, Ryan JM, Tobin L, et al. Cell contact, prostaglandin E (2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25 (High) forkhead box P3+ regulatory T cells.Clin Exp Immunol 2009; 156: 149-160. (back)
- Zhang Q, Shi S, Liu Y, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol 2009; 183: 7787-7798. (back)
- Sioud M, Mobergslien A, Boudabous A, Fløisand Y. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol 2010; 71: 267-274. (back)
- Madec AM, Mallone R, Afonso G et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells.Diabetologia 2009; 52: 1391-1399. (back)
- González MA, Gonzalez-Rey E, Rico L, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 2009; 60: 1006-1019. (back)
- Gonzalez-Rey E, Gonzalez MA, Varela N, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis 2010; 69: 241-248. (back)
- Najar M, Rouas R, Raicevic G, et al. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: the importance of low cell ratio and role of interleukin-6. Cytotherapy 2009; 11: 570-583. (back)
- Crop M, Baan CC, Korevaar SS, et al. Human adipose tissue-derived mesenchymal stem cells induce explosive T-cell proliferation. Stem Cells Dev 2010; 19: 1843-1853. (back)
- Najar M, Rouas R, Raicevic G, et al. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: the importance of low cell ratio and role of interleukin-6. Cytotherapy 2009; 11: 570-583. (back)
- Ge W, Jiang J, Baroja ML, et al. Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant 2009; 9: 1760-1772. (back)
- Hoogduijn MJ, Crop MJ, Peeters AM, et al. Donor-derived mesenchymal stem cells remain present and functional in the transplanted human heart. Am J Transplant 2009; 9: 222-230. (back)
- Bartholomew A, Sturgeon C, Siatskas M et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30:42-48. (back)
- Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 2010; 28: 585-596. (back)
- Sensebé L, Krampera M, Schrezenmeier H, et al. Mesenchymal stem cells for clinical application. Vox Sang 2010; 98: 93-107. (back)
- Shi M. and Liu Z.W. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol 2011; 164: 1-8. (back)
- Ning H, Yang F, Jiang M, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia 2008; 22: 593-599. (back)
- Vianello F, Dazzi F. Mesenchymal stem cells for graft-versus-host disease: a double edged sword? Leukemia 2008; 22: 463-465. (back)
- Liang J, Zhang H, Hua B, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis 2010; 69: 1423-1429. (back)
- Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature 1984; 307: 168-170. (back)
- Wekerle T, Kurtz J, Ito H, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med 2000; 6: 464-469. (back)
- Fandrich F, Dresske B, Bader M, Schulze M. Embryonic stem cells share immune-privileged features relevant for tolerance induction. J Mol Med 2002; 80: 343-350. (back)
- Bartholomew A, Sturgeon C, Siatskas M et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30:42-48. (back)
- Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003; 57: 11-20. (back)
- Potian JA, Aviv H, Ponzio NM, et al. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol 2003; 171: 3426-3434. (back)
- Bartholomew A, Sturgeon C, Siatskas M et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30:42-48. (back)
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003; 101: 3722-3729. (back)
- Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004; 363: 1439-1441. (back)
- Ringdén O; Uzunel M; Rasmusson I, et al. Mesenchymal Stem Cells for Treatment of Therapy-Resistant Graft-versus-Host Disease. Transplantation 2006; 81: 1390-1397. (back)
- 52 (back)
- Pawelec G, Rehbein A, Schlotz E, et al. Cytokine modulation of TH1/TH2 phenotype differentiation in directly alloresponsive CD4+ human T cells. Transplantation 1996; 62: 1095-1101. (back)
- Pawelec G, Rehbein A, Schlotz E, et al. Cytokine modulation of TH1/TH2 phenotype differentiation in directly alloresponsive CD4+ human T cells. Transplantation 1996; 62: 1095-1101. (back)
- Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol 2003; 3: 199-210. (back)
- Niederkorn JY, Peeler JS, Ross J, Callanan D. The immunogenic privilege of corneal allografts. Reg Immunol 1989; 2: 117-124. (back)
- Niederkorn JY, Peeler JS, Ross J, Callanan D. The immunogenic privilege of corneal allografts. Reg Immunol 1989; 2: 117-124. (back)
- van den Eynde B, Gaugler B, van der Bruggen P, et al. Human tumor antigens recognised by T cells: perspectives for new cancer vaccines. Biochem Soc Transact 1995; 23: 681-686. (back)
- Koc ON, Day J, Nieder M, et al. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant 2002; 30: 215-222. (back)
- Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18: 307-316. (back)
- Horwitz EM, Prockop DJ, Gordon PL, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood 2001; 97: 1227-1231. (back)
- Saito T, Kuang JQ, Bittira B, et al. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg 2002; 74: 19-24. (back)
- Deng W, Han Q, Liao L, et al. Allogeneic bone marrow-derived flk-1+Sca-1- mesenchymal stem cells leads to stable mixed chimerism and donor-specific tolerance. Exp Hematol 2004; 32: 861-867. (back)
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815-1822. (back)
- Potian JA, Aviv H, Ponzio NM, et al. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol 2003; 171: 3426-3434. (back)
- Bartholomew A, Sturgeon C, Siatskas M et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30:42-48. (back)
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003; 101: 3722-3729. (back)
- Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev 2004; 13: 263-271. (back)
- Deng W, Han Q, Liao L, et al. Allogeneic bone marrow-derived flk-1+Sca-1- mesenchymal stem cells leads to stable mixed chimerism and donor-specific tolerance. Exp Hematol 2004; 32: 861-867. (back)
- Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005; 105: 4120-4126. (back)
- Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 2003; 31: 890-896. (back)
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003; 101: 3722-3729. (back)
- Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003; 75: 389-397. (back)
- Bartholomew A, Sturgeon C, Siatskas M et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30:42-48. (back)
- Carlin LM, Eleme K, McCann FE, Davis DM. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J Exp Med 2001; 194: 1507-1517. (back)
- Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: Membrane nanotubes connect immune cells. J Immunol 2004; 173: 1511-1513. (back)
- Vanherberghen B, Andersson K, Carlin LM, et al. Human and murine inhibitory natural killer cell receptors transfer from natural killer cells to target cells. Proc Natl Acad Sci U S A 2004; 101: 16873-16878. (back)
- Hunt JS, Petroff MG, Morales P, et al. HLA-G in reproduction: studies on the maternal-fetal interface. Hum Immunol 2000; 61: 1113-1117. (back)
- Ristich V, Liang S, Zhang W, et al. Tolerization of dendritic cells by HLA-G. Eur J Immunol 2005; 35: 1133-1142. (back)
- Moretta L, Moretta A. Killer immunoglobulin-like receptors. Curr Opin Immunol 2004; 16: 626-633. (back)
- Parham P. Killer cell immunoglobulin-like receptor diversity: balancing signals in the natural killer cell response. Immunol Lett 2004; 92: 11-13. (back)
- Gomez-Lozano N, de Pablo R, Puente S, Vilches C. Recognition of HLA-G by the NK cell receptor KIR2DL4 is not essential for human reproduction. Eur J Immunol 2003; 33: 639-644. (back)
- Gotherstrom C, Ringden O, Tammik C, et al. mmunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol 2004; 190: 239-245. (back)
- Majumdar MK, Keane-Moore M, Buyaner D, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci 2003; 10: 228-241. (back)
- Devine SM, Hoffman R. Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol 2000; 7: 358-363. (back)
- Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003; 75: 389-397. (back)
- Majumdar MK, Keane-Moore M, Buyaner D, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci 2003; 10: 228-241. (back)
- Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003; 102: 3837-3844. (back)
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003; 101: 3722-3729. (back)
- Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005; 105: 2214-2219. (back)
- Di Nicola M, Carlo-Stella M C, Magni, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838-3843. (back)
- Guinan EC, Gribben JG, Boussiotis VA, et al. Pivotal role of the B7:CD28 pathway in transplantation tolerance and tumor immunity. Blood 1994; 84: 3261-3282. (back)
- Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A 2002; 99: 351-358. (back)
- Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A 2002; 99: 351-358. (back)
- Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A 2002; 99: 351-358. (back)
- Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A 2002; 99: 351-358. (back)
- Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol 2004; 4: 841-855. (back)
- Niederkorn JY, Peeler JS, Ross J, Callanan D. The immunogenic privilege of corneal allografts. Reg Immunol 1989; 2: 117-124. (back)
- Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005; 105: 2214-2219. (back)
- Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005; 105: 4120-4126. (back)
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003; 101: 3722-3729. (back)
- Bartholomew A, Sturgeon C, Siatskas M et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30:42-48. (back)
- Niederkorn JY, Peeler JS, Ross J, Callanan D. The immunogenic privilege of corneal allografts. Reg Immunol 1989; 2: 117-124. (back)
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 1991; 9: 271-296. (back)
- Steinman RM, Inaba K, Turley S, et al. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum Immunol 1999; 60: 562-567. (back)
- Mahon BP, Katrak K, Nomoto A, et al. Poliovirus-specific CD4+ Th1 clones with both cytotoxic and helper activity mediate protective humoral immunity against a lethal poliovirus infection in transgenic mice expressing the human poliovirus receptor. J Exp Med 1995; 181: 1285-1292. (back)
- Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol 2004; 4: 841-855. (back)
- Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol 2004; 4: 408-414. (back)
- Di Nicola M, Carlo-Stella M C, Magni, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838-3843. (back)
- Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003; 75: 389-397. (back)
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815-1822. (back)
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815-1822. (back)
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815-1822. (back)
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003; 101: 3722-3729. (back)
- Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005; 105: 2214-2219. (back)
- Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005; 105: 2821-2827. (back)
- Wells AD, Walsh MC, Bluestone JA, Turka LA. Signaling through CD28 and CTLA-4 controls two distinct forms of T cell anergy. J Clin Invest 2001; 108: 895-903. (back)
- Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005; 105: 2821-2827. (back)
- Otten GR, Germain RN. Split anergy in a CD8+ T cell: receptor-dependent cytolysis in the absence of interleukin-2 production. Science 1991; 251: 1228-1231. (back)
- Nash AA, Ashford NP. Split T-cell tolerance in herpes simplex virus-infected mice and its implication for anti-viral immunity. Immunology 1982; 45: 761-767. (back)
- Martinez C, Smith JM. Split Tolerance. Nature 1964; 202: 508-509. (back)
- Masson D, Tschopp J. A family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell 1987; 49: 679-685. (back)
- Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 1990; 11: 237-244. (back)
- Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 1990; 11: 237-244. (back)
- Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 2003; 76: 1208-1213. (back)
- Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol 2004; 4: 408-414. (back)
- Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 2003; 76: 1208-1213. (back)
- Dormady SP, Bashayan O, Dougherty R, et al. Immortalized multipotential mesenchymal cells and the hematopoietic microenvironment. J Hematother Stem Cell Res 2001; 10: 125-140. (back)
- Zhang Y, Li CD, Jiang XX, et al. Comparison of mesenchymal stem cells from human placenta and bone marrow. Chin Med J [Engl] 2004; 117: 882-887. (back)
- Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol 1996; 166: 585-592. (back)
- Silva WAJ, Covas DT, Panepucci RA, et al. The profile of gene expression of human marrow mesenchymal stem cells. Stem Cells 2003; 21: 661-669 (back)
- Kuroiwa T, Kakishita E, Hamano T, et al. Hepatocyte growth factor ameliorates acute graft-versus-host disease and promotes hematopoietic function. J Clin Invest 2001; 107: 1365-1373. (back)
- Taniguchi F, Harada T, Deura I, et al. Hepatocyte growth factor promotes cell proliferation and inhibits progesterone secretion via PKA and MAPK pathways in a human granulosa cell line. Mol Reprod Dev 2004; 68: 335-344. (back)
- Xin X, Yang S, Ingle G, et al. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol 2001; 158: 1111-1120. (back)
- Xin X, Yang S, Ingle G, et al. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol 2001; 158: 1111-1120. (back)
- Chunmeng S, Tianmin C, Yongping S, et al. Effects of dermal multipotent cell transplantation on skin wound healing. J Surg Res 2004; 121: 13-19. (back)
- Ono I, Yamashita T, Hida T, et al. Local administration of hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. J Surg Res 2004; 120: 47-55. (back)
- Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 2003; 102: 3837-3844. (back)
- Di Nicola M, Carlo-Stella C, Magni M et al. Human bone marrow stromal cells suppress T lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838-3843. (back)
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 2003; 101: 3722-3729. (back)
- Chunmeng Le Blanc K, Rasmusson I, Gotherstrom C, et al. Mesenchymal stem cells inhibit the expression of CD25 [interleukin-2 receptor] and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol 2004; 60: 307-315. (back)
- Neuss S, Becher E, Woltje M, et al. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells 2004; 22: 405-414. (back)
- Di Nicola M, Carlo-Stella C, Magni M et al. Human bone marrow stromal cells suppress T lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838-3843. (back)
- Barry FP, Murphy JM, English K, Mahon BP. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cell Dev 2005; 14: 252-265. (back)
- Chunmeng Le Blanc K, Rasmusson I, Gotherstrom C, et al. Mesenchymal stem cells inhibit the expression of CD25 [interleukin-2 receptor] and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol 2004; 60: 307-315. (back)
- Neuss S, Becher E, Woltje M, et al. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells 2004; 22: 405-414. (back)
- Neuss S, Becher E, Woltje M, et al. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells 2004; 22: 405-414. (back)
- Ono I, Yamashita T, Hida T, et al. Local administration of hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. J Surg Res 2004; 120: 47-55. (back)
- Azuma H, Takahara S, Matsumoto K, et al. Hepatocyte growth factor prevents the development of chronic allograft nephropathy in rats. J Am Soc Nephrol 2001; 12: 1280-1292. (back)
- Asseman C, Powrie F. Interleukin 10 is a growth factor for a population of regulatory T cells. Gut 1998; 42: 157-158. (back)
- Ennis DP, Cassidy JP, Mahon BP. Prior Bordetella pertussis infection modulates allergen priming and the severity of airway pathology in a murine model of allergic asthma. Clin Exp Allergy 2004; 34: 1488-1497. (back)
- Mahon BP, Ryan M, Griffin F, Mills KH. IL-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting the induction of Th1 cells. Infect Immun 1996; 64: 5295-5301. (back)
- Flynn MA, Casey DG, Todryk SM, Mahon BP. Efficient delivery of small interfering RNA for inhibition of IL-12p40 expression in vivo. J Inflamm (Lond) 2004; 1: 4. (back)
- Pretolani M, Goldman M. IL-10: a potential therapy for allergic inflammation. Immunol Today 1997 Mahon BP, Ryan M, Griffin F, 18: 277-280. (back)
- Higgins SC, Lavelle EC, McCann C, et al. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J Immunol 2003; 171: 3119-3127. (back)
- McGuirk P, Mills KHG. Direct anti-inflammatory effect of a bacterial virulence factor:IL-10-dependent suppression of IL-12 production by filamentous haemagglutinin from Bordetella pertussis. Eur J Immunol 2000; 30: 415-422. (back)
- 112 (back)
- Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003; 75: 389-397. (back)
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815-1822. (back)
- Arikawa T, Omura K, Morita I. Regulation of bone morphogenetic protein-2 expression by endogenous prostaglandin E2 in human mesenchymal stem cells. J Cell Physiol 2004; 200: 400-406. (back)
- Roper RL, Ludlow JW, Phipps RP. Prostaglandin E2 inhibits B lymphocyte activation by a cAMP-dependent mechanism: PGE-inducible regulatory proteins. Cell Immunol 1994; 154: 296-308. (back)
- Akasaki Y, Liu G, Chung NH, et al. Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. J Immunol 2004; 173: 4352-4359. (back)
- Strand S, Galle PR. Immune evasion by tumours: involvement of the CD95 [APO-1/Fas] system and its clinical implications. Mol Med Today 1998; 4: 63-68. (back)
- Mitra R, Singh S, Khar A. Antitumour immune responses. Expert Rev Mol Med 2003; 5: 1-19. (back)
- Mitra R, Singh S, Khar A. Antitumour immune responses. Expert Rev Mol Med 2003; 5: 1-19. (back)
- Guinan EC, Gribben JG, Boussiotis VA, et al. Pivotal role of the B7:CD28 pathway in transplantation tolerance and tumor immunity. Blood 1994; 84: 3261-3282. (back)
- Fujiwara K, Higashi T, Nouso K, et al. Decreased expression of B7 costimulatory molecules and major histocompatibility complex class-I in human hepatocellular carcinoma. J Gastroenterol Hepatol 2004; 19: 1121-1127. (back)
- Jung D, Hilmes C, Knuth A, Jaeger E, et al. Gene transfer of the Co-stimulatory molecules B7-1 and B7-2 enhances the immunogenicity of human renal cell carcinoma to a different extent. Scand J Immunol 1999; 50: 242-249. (back)
- Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003; 75: 389-397. (back)
- Majumdar MK, Keane-Moore M, Buyaner D, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci 2003; 10: 228-241. (back)
- Ninomiya T, Akbar SM, Masumoto T, et al. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol 1999; 31: 323-331. (back)
- Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood 2005; 105: 2214-2219. (back)
- Rosenberg SA. The development of new immunotherapies for the treatment of cancer using interleukin-2. A review. Ann Surg 1988; 208: 121-135. (back)
- Chunmeng Le Blanc K, Rasmusson I, Gotherstrom C, et al. Mesenchymal stem cells inhibit the expression of CD25 [interleukin-2 receptor] and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol 2004; 60: 307-315. (back)
- Chunmeng Le Blanc K, Rasmusson I, Gotherstrom C, et al. Mesenchymal stem cells inhibit the expression of CD25 [interleukin-2 receptor] and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol 2004; 60: 307-315. (back)
- Bartholomew A, Sturgeon C, Siatskas M et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol 2002; 30:42-48. (back)
- Chunmeng Le Blanc K, Rasmusson I, Gotherstrom C, et al. Mesenchymal stem cells inhibit the expression of CD25 [interleukin-2 receptor] and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol 2004; 60: 307-315. (back)
- Nakamura K, Kitani A, Fuss I, et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol 2004; 172: 834-842. (back)
- Ranges GE, Figari IS, Espevik T, Palladino MAJ. Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med 1987; 166: 991-998. (back)
- van der Pouw Kraan TC, Boeije LC, Snijders A, et al. Regulation of IL-12 production by human monocytes and the influence of prostaglandin E2. Ann N Y Acad Sci 1996; 795: 147-157. (back)
- Mahon BP, Ryan M, Griffin F, Mills KH. IL-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting the induction of Th1 cells. Infect Immun 1996; 64: 5295-5301. (back)
- Mitra R, Singh S, Khar A. Antitumour immune responses. Expert Rev Mol Med 2003; 5: 1-19. (back)
- Chang HL, Gillett N, Figari I, et al. Increased transforming growth factor beta expression inhibits cell proliferation in vitro, yet increases tumorigenicity and tumor growth of Meth A sarcoma cells. Cancer Res 1993; 53: 4391-4398. (back)
- Ryan JM, Barry FP, Murphy MJ, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005; 26: 2: 8. (back)
- Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol 2004; 22: 1136-1151. (back)
- Casiraghi F, Azzollini N, Cassis P, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol 2008; 181: 3933-3946. (back)
- Casiraghi F, Azzollini N, Todeschini M, et al. Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. Am J Transplant 2012; 12: 2373-2383. (back)
- Ryan JM, Barry FP, Murphy MJ, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005; 26: 2: 8. (back)
- Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol 2004; 22: 1136-1151. (back)
- Casiraghi F, Azzollini N, Cassis P, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol 2008; 181: 3933-3946. (back)
- Casiraghi F, Azzollini N, Todeschini M, et al. Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. Am J Transplant 2012; 12: 2373-2383. (back)
- Vanikar AV, Trivedi HL. Stem cell transplantation in living donor renal transplantation for minimization of immunosuppression. Transplantation 2012; 94: 845-850. (back)
- Peng Y, Ke M, Xu L, Liu L, Chen X, Xia W, Li X, Chen Z, Ma J, Liao D, Li G, Fang J, Pan G, Xiang AP. Donor-derived mesenchymal stem cells combined with low-dose tacrolimus prevent acute rejection after renal transplantation: a clinical pilot study. Transplantation 2013; 95: 161-168. (back)
- Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, Pileggi A, Ricordi C. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA 2012; 307: 1169-1177. (back)
- Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, Claas FH, van Miert PP, Roelen DL, van Kooten C, Fibbe WE, Rabelink TJ. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase i study. Stem Cells Transl Med 2013; 2: 107-111. (back)
- Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, Claas FH, van Miert PP, Roelen DL, van Kooten C, Fibbe WE, Rabelink TJ. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase i study. Stem Cells Transl Med 2013; 2: 107-111. (back)
To cite this article
Mesenchymal Stem Cells And Solid Organ Transplantation
CellR4 2013; 1 (2): e377
Publication History
Published online: 30 Sep 2013

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.