CellR4 2020;
8: e2846
DOI: 10.32113/cellr4_20205_2846
Editorial – Potential risks of the SARS-related coronavirus 2 infection [COVID-19] in pregnancy
Topic: COVID-19, Infectious Diseases
Category: Editorials
Abstract
Introduction
At the end of 2019, a cluster of atypical pneumonia cases in Wuhan, China was reported to the World Health Organization (WHO)1. These cases were associated with severe respiratory failure and were quickly found to be caused by a novel coronavirus, likely originated in bats2. Coronavirus belongs to the coronaviridae family and is a single stranded RNA-enveloped virus. The new virus would be named the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Coronavirus disease 2019 (COVID-19), as the syndrome became known, spread quickly with lethal results, and was declared a Public Health Emergency of International Concern on 30 January 2020 by the WHO. SARS-CoV-2 gains entry into cells via the same angiotension-converting enzyme 2 (ACE2) receptor as other coronaviruses3 [Figure 1]. ACE2 is expressed in type II alveolar cells in the respiratory tract, as well as multiple other sites including the GI tract, and has cross species reactivity likely explaining the jump from bat to human4.
COVID-19 manifests as mild fever with or without cough, myalgia, headache, diarrhea, respiratory decompensation, and death. The majority of cases are mild, however severe disease is reported in 5-10%, with a case fatality rate much higher than for previously described similar viruses5. It appears that the most severe cases are related to delayed and excessive inflammatory reaction related to a “cytokine storm” and leading to acute respiratory distress syndrome (ARDS) and multi-organ failure6. The initial response in the obstetric community was heightened concern, given that previous coronavirus outbreaks were associated with severe adverse maternal and fetal outcomes, including a high risk of death7, 8. However the initial reports suggested that outcomes in COVID-19 were similar in pregnant and nonpregnant individuals9. While this is reassuring, COVID-19 can manifest as a severe disease, which is associated with significant morbidity and mortality.
This editorial summarizes the available information regarding COVID-19 from a maternal and fetal perspective, with emphasis on underlying mechanisms of disease, and thus exploring future directions for evaluation and management.
Maternal Perspective
Pregnancy is a hyperdynamic state with profound changes from baseline non-pregnant physiology, necessary to accommodate metabolic and other needs of the mother and growing fetus10. Respiratory drive increases resulting in hyperventilation and mild respiratory alkalosis (pH 7.44 vs. 7.40 in non-pregnant state) 11. Tidal volume, peak expiratory flow rates and airway conductance increase, while total lung capacity (TLC) decreases 10,11. The carbon-dioxide (CO2) partial pressure decreases to ensure maximum oxygen delivery to maternal lungs and optimal CO2 transfer/exchange from fetus to mother. As gestation progresses, the gravid uterus expands cephalad beyond the umbilicus, resulting in upward displacement of the diaphragm by approximately 4 cm10.
The physiological adaptions of the immune system which balance maternal protection from the environment with fetal protection from the mother, involve complex interaction with maternal and fetal components: microbiome, uterine decidua and trophoblast 10,12. Proposed mechanisms of immune suppression include placental mechanical barrier, systemic immune suppression, absence of major histocompatibilty complex (MHC) class 1 molecules on trophoblasts, cytokine shifts from Th1 to Th2 depending on trimester, and local immune suppresion mediated by Fas/FasL ligand system. A relative proinflammatory state (Th1 dominance) in the first trimester of pregnancy promotes decidual trophoblast invasion with neovascularization and development of the maternal fetal intervillous area needed for exchange of gases and nutrients. Second trimester converts to an anti-inflammatory state (Th2 dominance), allowing for fetal growth and development. The third trimester partially reverts to a pro-inflammatory (Th1) state secondary to influx of immune cells into the myometrium in preparation for parturition. Expression of MHC on trophoblast also allows immune tolerance and fetal protection, with the placenta expressing a non-classic MHC form HLA-E, F, G, recognized by natural killer cells (NK cells) in decidua and inhibiting their activity 12.
The sum changes during pregnancy place the mother and fetus at varying risk from COVID-19 and similar illnesses based on gestational age. The added increased oxygen demand and decreased TLC in pregnancy can result in subsequent decrease in alveolar-capillary exchange and rapid respiratory compromise13. The pro-inflammatory first and third trimester stages can be triggered by viral pathogens, and this is reflected in the current literature, with most reports of severe COVID-19 during pregnancy presenting in the third trimester14. Early pregnancy loss (miscarriage) has been reported with the coronaviruses, but is not well studied and represents a target for more research (see below)7, 8.
In the largest US series of COVID-19 in pregnancy, 86% of women had mild disease, 9.3% had severe disease and 4.7% had critical disease15. This series was the first to report on a universal screening protocol and included both asymptomatic and symptomatic patients. All women recovered. A recent systematic review summarized the global available literature on COVID-19 in pregnancy14. They reported 108 pregnancies until April 4, 2020. Most women presented with fever and cough in the third trimester; those presenting in early pregnancy had mild disease and were discharged without delivery. ICU admission was reported in 3 patients (3%). Lymphocytopenia and elevated C-reactive protein (a marker of inflammation) were noted in the majority of patients. All patients recovered. The finding of generally benign disease in pregnancy has surprised the community and may be related to the relatively anti-inflammatory state assocciated with Th2 predominance of the immune system.

Figure 1. (A) In type II pneumocytes, Angiotensin II (Ang II) is converted to Angiotensin-(1-7) [(Ang-(1-7)] via the angiotensin-converting enzyme 2 receptor (ACE2). Ang-(1-7) can bind to other receptors leading to vasodilation and attenuation of an inflammatory response. Ang II can also bind to Angiotensin II Type 1 Receptor (AT1R) resulting in vasoconstriction and increased oxidative stress thus promoting a pro-inflammatory response and fibrosis. (B) SARS-CoV-2 binds to ACE2 gaining entry to type II pneumocytes. After endocytosis of the viral complex, ACE2 expression on the host cell is down-regulated, resulting in unopposed accumulation of Ang II, which bind AT1R and potentially leads to acute lung injury.
Fetal Perspective
Fetal effects in pregnancy occur through two main mechanisms. The first involves transmission of the virus to the fetus, either through ascending organisms from the genital tract, direct inoculation from the abdominal cavity, or transplacental infection via the maternal blood stream; transmission may also occur through breastmilk. Transmission may result in congenital anomalies, or chronic infection in the child, known as vertical transmission, or mother-to-child transmission (MTCT). The risk of congenital anomalies is classically reported to be highest with teratogen exposure in the first trimester during the period of organogenesis, however viruses may act through a destructive pathway, whereby a normally formed organ is damaged by subsequent viral infection. The second avenue by which the fetus is affected is through maternal disease: severe illness in the mother may cause uteroplacental compromise or preterm labor, or maternal disease may lead to early delivery putting the fetus at risk of prematurity and its complications.
Most maternal viral infections are not transmitted to the fetus, likely due to the immune changes described above16. As an example, influenza, caused by an RNA virus, is known to cause severe maternal disease, with a high risk of life-threatening pneumonia, however it has not been associated with congenital anomalies or vertical transmission. In contrast, viruses such as varicella-zoster virus (VZV), rubella, parvovirus, cytomegalovirus (CMV) and Zika virus have the potential to cause devastating fetal infection, especially when contracted in the first trimester, leading to severe congenital anomalies17 and pregnancy loss18. As an example, first trimester VZV infection is associated with chorioretinitis, microphthalmia, cerebral cortical atrophy, growth restriction, limb hypoplasia and skin lesions19. Primary CMV in pregnancy has profound fetal effects including growth restriction, microcephaly, intracranial calcifications, hematologic abnormalities and neurologic deficits. Zika virus is associated with a mainly destructive process, leading to microcephaly and severe neurologic abnormalities. Multiple viruses are associated with vertical transmission, including hepatitis B virus and HIV. Maternal and neonatal therapies have been shown to reduce the transmission of these viruses20, 21.
To date, none of the coronaviruses have been associated with congenital anomalies. Vertical transmission has not been definitively demonstrated. In COVID-19 viremia is rare, therefore transmission through this mechanism is doubtful22. In the series mentioned above, 1 case (out of 75) of suspected transmission was described, however the infant testing was performed at 36 hours of life, thus a postnatal infection cannot be excluded14. Subsequently 3 cases were reported of SARS-CoV-2 positive mothers, whose newborns had elevated IgG and IgM levels for COVID-19 hours after birth23, 24. While IgG antibodies cross the placenta and may be of maternal origin, IgM antibodies are not known to pass the placental barrier and therefore are presumed to be a fetal reaction. The neonates reported in these studies all tested negative for SARS-CoV-2, and there was no cord blood or placenta collection for analysis. Given that IgM antibodies are known to cross-react, these reports cannot be used to conclude that vertical transmission occurred. There is limited information regarding transmission in breastmilk. In a study of 9 women with COVID-19, all breastmilk samples tested negative for the virus25. This is an active area of interest and is one of our focuses in particular.
It appears that one of the main issues thus far with COVID-19 in pregnancy is that severe maternal disease is associated with relative fetal decompensation, resulting in fetal heart rate decelerations which prompt urgent delivery. The largest series to date of pregnancies with COVID-19 reported a 92% cesarean delivery rate, most commonly due to “fetal distress”14. In many reports these deliveries are preterm and are associated with neonatal intensive care unit (NICU) admissions. Thus neonatal morbidity seems to be more related to prematurity rather than the COVID-19 diagnosis per se.
Future Directions
The approach to containment of the COVID-19 pandemic has focused on a global, coordinated public health effort to mitigate active spread of disease. Much remains to be learned about COVID-19, both in pregnancy and outside of pregnancy. We and others are gathering data in order to more accurately describe the epidemiology and seroprevalence of the disease. It is important to note that the majority of cases reported thus far are largely cases of infection in the third trimester. We have yet to see the effects of this virus on the fetus when it affects the mother in the first trimester. As discussed earlier, many viruses capable of causing fetal malformation, spontaneous abortion or stillbirth do so when they are contracted in the first trimester. Whether or not vertical transmission, and/or transmission through breastmilk, occurs also remains an open question.
Until an effective vaccine can be developed, emphasis going forward will be placed on optimizing treatment. A recent review summarizes the pharmacologic approach to COVID-19 including ‘repurposed’ agents and investigational agents, including a discussion on the limited data regarding chloroquine and hydrochloroquine, two agents used successfully in pregnancy for other conditions26. Antiviral agents have been administered in other viral diseases in pregnancy, such as HIV, and antibiotics, such as azithromycin, are other agents under consideration for treatment in pregnancy. Two other areas deserve mention here.
- ACE2 mechanisms and signaling pathways – ACE2 counter regulates the renin-angiotensin system (RAS) by converting angiotensin II (Ang II) into Ang-(1-7), which leads to vasodilation and increased sodium excretion thereby lowering blood pressure27. ACE2 also acts via nitric oxide pathway to decrease inflammation28. SARS-Co-V is thought to bind ACE2 and suppress its expression, which could lead to lung damage/inflammation via Ang II accumulation29 and a similar mechanism can be proposed for SARS-CoV-2 (which binds to ACE2 via its spike protein)27. What effect ACE inhibitors and/or angiotensin receptor blockers (ARBs) have on COVID-19, if any, is unclear and is actively being studied30. In fact “competing hypothetical mechanisms” have been proposed, whereby on the one side, RAS inhibition leads to worse COVID-19 disease, and on the other it could theoretically improve it27, 30. While ACE inhibitors and ARBs are not used during pregnancy because of adverse fetal effects, many women who become ill with COVID-19 are diagnosed in the peripartum period when their use is permissible. A therapeutic targeting this pathway could certainly be used in the peripartum period (with awareness of lactation status).
- Anti-inflammatory and immunomodulatory agents – Given the evidence of a cytokine storm playing a key role in the pathogenesis of severe COVID-19, immunomodulatory treatment could be beneficial. Empiric evidence from other respiratory viral disease outbreaks has demonstrated worse outcomes with immunosuppressive agents and anecdotal reports suggest the same could be true with COVID-19. A systematic review demonstrated a lack of adequate studies examining these therapies in COVID-1931. Fortunately a number of clinical trials are underway examining monoclonal antibodies such as tocilizumab, sarilumab, and siltuximab against IL-6 as adjunctive therapies [Figure 2]26, 32. Finally, convalescent plasma of people who have recovered from COVID-19 is a possible therapy, having been used in previous coronavirus outbreaks with some success33.
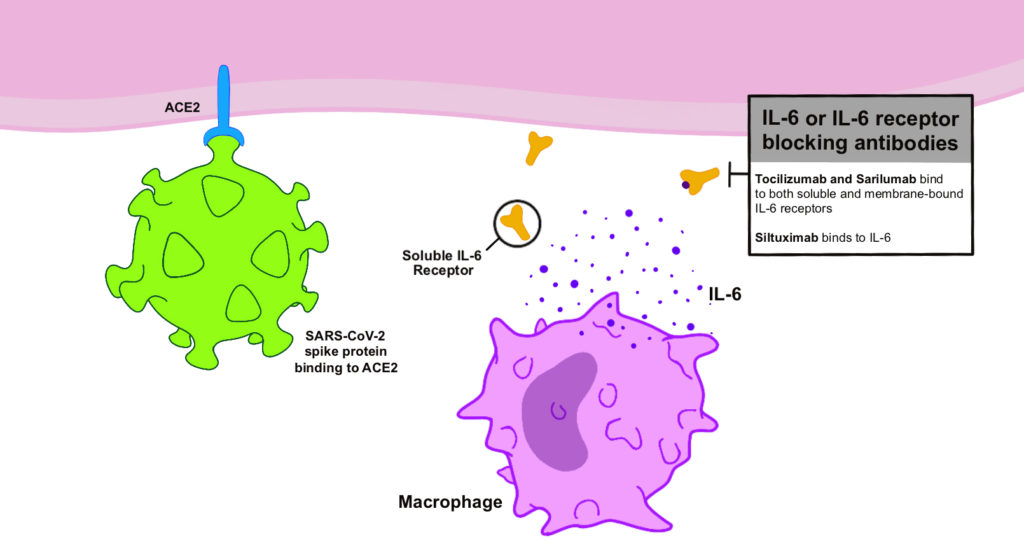
Figure 2. SARS-CoV-2 has bound to the cell via the ACE2 receptor. The mechanism of action of IL-6 or IL-6 receptor blocking antibodies is demonstrated as possible target to combat the “cytokine storm” associated with COVID-19.
At the time of this writing, in the US there were 1,477,516 total COVID-19 cases and 89,272 total deaths, while worldwide the numbers were 4,735,622 confirmed cases, and 316,289 total deaths1. The true extent of the epidemic remains to be seen.
Conflict of Interest
The Authors declare that they have no conflict of interests.
Acknowledgements
Original artwork was created by Jacqueline M. Sanchez, MD, MS and Marina Zorrilla, MD, both OBGYN residents of the University of Miami/Jackson Health System Program.
References
[1] WHO. Coronavirus disease (COVID-19) Pandemic. World Health Organization.
[2] Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020, 395: 565-574.
[3] Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579: 270-273.
[4] Li R, Qiao S, Zhang G. Analysis of angiotensin-converting enzyme 2 (ACE2) from different species sheds some light on cross-species receptor usage of a novel coronavirus 2019-nCoV. J Infect 2020; 80: 469-496.
[5] Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020 Feb 24. doi: 10.1001/jama.2020.2648. Epub ahead of print.
[6] Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect 2020; 80: 607-613.
[7] Schwartz DA. An Analysis of 38 Pregnant Women with COVID-19, Their Newborn Infants, and Maternal-Fetal Transmission of SARS-CoV-2: Maternal Coronavirus Infections and Pregnancy Outcomes. Arch Pathol Lab Med. 2020 Mar 17. doi: 10.5858/arpa.2020-0901-SA. Epub ahead of print.
[8] Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, Vecchiet J, Nappi L, Scambia G, Berghella V, D’Antonio F. Outcome of Coronavirus spectrum infections (SARS, MERS, COVID 1 -19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 020 Mar 25:100107. doi: 10.1016/j.ajogmf.2020.100107. Epub ahead of print.
[9] Liu D, Li L, Wu X, Zheng D, Wang J, Yang L, Zheng C. Pregnancy and Perinatal Outcomes of Women With Coronavirus Disease (COVID-19) Pneumonia: A Preliminary Analysis. Am J Roentgenol 2020: 1-6.
[10] Cunningham FG, Leveno KJ, Bloom SL, Dashe JS, Hoffman BL, Casey BM, Spong CY. Maternal Physiology. Williams Obstetrics, 25e. New York, NY: McGraw-Hill Education, 2018.
[11] McAuliffe F, Kametas N, Costello J, Rafferty GF, Greenough A, Nicolaides K. Respiratory function in singleton and twin pregnancy. BJOG 2002; 109: 765-769.
[12] Fuhler GM. The immune system and microbiome in pregnancy. Best Pract Res Clin Gastroenterol 2020; 44-45: 101671.
[13] Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li S, Biswas A, Choolani M, Mattar C, Su LL. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol 2020; S0002-9378(20)30343-4.
[14] Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet Gynecol Scand 2020 Apr 7. doi: 10.1111/aogs.13867. Epub ahead of print.
[15] Breslin N, Baptiste C, Gyamfi-Bannerman C, Miller R, Martinez R, Bernstein K, Ring L, Landau R, Purisch S, Friedman AM, Fuchs K, Sutton D, Andrikopoulou M, Rupley D, Sheen JJ, Aubey J, Zork N, Moroz L, Mourad M, Wapner R, Simpson LL, D’Alton ME, Goffman D. COVID-19 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM 2020 Apr 9:100118. doi: 10.1016/j.ajogmf.2020.100118. Epub ahead of print.
[16] Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th Edition ed. Philadelphia: Saunders/Elsevier, 2019.
[17] Chakhtoura N, Hazra R, Spong CY. Zika virus: a public health perspective. Curr Opin Obstet Gynecol 2018; 30: 116-122.
[18] Pereira L. Congenital viral infection: traversing the uterine-placental interface. Annu Rev Virol 2018; 5: 273-299.
[19] Lamont RF, Sobel JD, Carrington D, Mazaki-Tovi S, Kusanovic JP, Vaisbuch E, Romero R. Varicella-zoster virus (chickenpox) infection in pregnancy. BJOG 2011; 118: 1155-62.
[20] Gentile I, Borgia G. Vertical transmission of hepatitis B virus: challenges and solutions. Int J Womens Health 2014, 6: 605-611.
[21] Bailey H, Zash R, Rasi V, Thorne C. HIV treatment in pregnancy. Lancet HIV 2018; 5: e457-e467.
[22] Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020; 323: 1843-1844.
[23] Dong L, Tian J, He S, Zhu C, Wang J, Liu C, Yang J. Possible Vertical Transmission of SARS-CoV-2 From an Infected Mother to Her Newborn. JAMA 2020; 323: 1846-1848.
[24] Zeng H, Xu C, Fan J, Tang Y, Deng Q, Zhang W, Long X. Antibodies in Infants Born to Mothers With COVID-19 Pneumonia. JAMA 2020; 323: 1848-1849.
[25] Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020; 395: 809-815.
[26] Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020 Apr 13. doi: 10.1001/jama.2020.6019. Online ahead of print.
[27] Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med 2020; 382: 1653-1659.
[28] Jin HY, Song B, Oudit GY, Davidge ST, Yu HM, Jiang YY, Gao PJ, Zhu DL, Ning G, Kassiri Z, Penninger JM, Zhong JC. ACE2 deficiency enhances angiotensin II-mediated aortic profilin-1 expression, inflammation and peroxynitrite production. PLoS One 2012; 7: e38502.
[29] Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005; 11: 875-879.
[30] South AM, Tomlinson L, Edmonston D, Hiremath S, Sparks MA. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol 2020; 16: 305-307.
[31] AminJafari A, Ghasemi S. The possible of immunotherapy for COVID-19: a systematic review. Int Immunopharmacol 2020; 83: 106455.
[32] Ascierto PA, Fox B, Urba W, Anderson AC, Atkins MB, Borden EC, Brahmer J, Butterfield LH, Cesano A, Chen D, de Gruijl T, Dillman RO, Drake CG, Emens LA, Gajewski TF, Gulley JL, Stephen Hodi F, Hwu P, Kaufman D, Kaufman H, Lotze M, McNeel DG, Margolin K, Marincola F, Mastrangelo MJ, Maus MV, Parkinson DR, Romero PJ, Sondel PM, Spranger S, Sznol M, Weiner GJ, Wiggington JM, Weber JS. Insights from immuno-oncology: the Society for Immunotherapy of Cancer Statement on access to IL-6-targeting therapies for COVID-19. J Immunother Cancer 2020; 8: e000878.
[33] Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, Makki S, Rooney KD, Nguyen-Van-Tam JS, Beck CR; Convalescent Plasma Study Group. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis 2015; 211: 80-90.
To cite this article
Editorial – Potential risks of the SARS-related coronavirus 2 infection [COVID-19] in pregnancy
CellR4 2020;
8: e2846
DOI: 10.32113/cellr4_20205_2846
Publication History
Submission date: 20 Apr 2020
Revised on: 12 May 2020
Accepted on: 25 May 2020
Published online: 27 May 2020

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.