CellR4 2014; 2 (4): e1090
Injectable Clostridium Histolyticum Collagenases for Mild Dupuytren’s Contracture: Preliminary Data
Category: Brief Communications
Abstract
BACKGROUND: Dupuytren’s disease is a connective tissue disorder affecting the hand, characterized by the formation of collagen-rich nodules and cords. It often results in shortening and thickening of the palmar fascia leading to permanent flexion contracture of the digits.
Nowadays surgery followed by hand therapy is standard treatment.
A new, non-surgical option is the injectable Clostridium histolyticum collagenase.
AIM: The purpose of our study is to extend the clinical use of collagenase injections as a nonsurgical therapy for Dupuytren’s disease.
MATERIALS AND METHODS: Intralesional use of Clostridial collagenase has been evaluated in our study in a total of four patients receiving injections, followed by cord fracture the day after injection, by finger extension procedure.
CONCLUSIONS: The study showed a significant reduction in contracture; treatment was well tolerated with self-limited adverse events.
INTRODUCTION
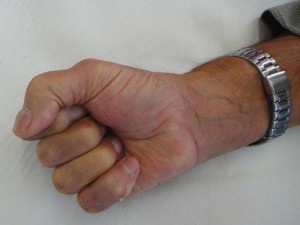
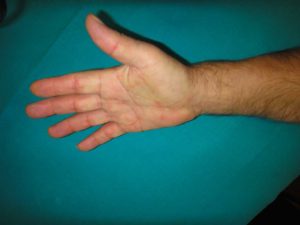
Dupuytren’s disease is a benign connective tissue disorder affecting the palmar fascia; it limits hand function and may disable the hand (Figure 1). It was first described by Felix Plater, a Swiss physician, in 1614 and later (1831) attributed to a French physician, Guillaume Dupuytren 1 , 2 , 3.
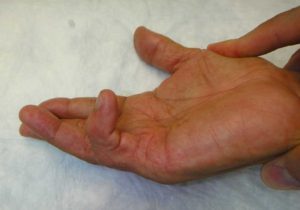
Figure 1. A patient with a right small finger metacarpophalangeal and proximal interphalangeal joint contracture.
Dupuytren’s Disease leads to progressive hand contractures: mediated by myofibroblasts, pathologic cords form and lead to progressive flexion deformity of the fingers, involving the metacarpophalangeal and proximal interphalangeal joint (Figures 2, 3, 4); deformity may become severe enough to interfere with daily living activities, cause embarrassment and consequently an impact on work abilities and quality of life 4 , 5 , 6.
Surgery is the mainstay of treatment and is recommended in patients with functional impairment and metacarpophalangeal-joint contractures of at least 30 degrees; however it is invasive, potentially involves a long recovery period and does not offer a definitive cure 7 , 8 , 9 , 10.
Various nonsurgical treatments (radiotherapy, physical therapy, topical vitamin A and E application, steroid injections, anti-gout medications, interferon-gamma, enzymatic treatments with trypsin, hyaluronidase, lidocaine) have been evaluated for Dupuytren’s Disease treatment; however were generally found to be ineffective or unsuitable for clinical use 11 , 12 , 13 , 14 , 15 , 16.
Abnormal synthesis of collagen leading to impaired joint function is the main problem of Dupuytren disease 17. Collagen as a target of treatment through collagenases is emerging as a nonsurgical treatment for Dupuytren’s contracture. A Clostridium histolyticum collagenase is injected into the affected cord without preliminary local anesthesia; the next day, the treated joint is manipulated to attempt cord rupture. Extensive hand therapy after treatment is not required 18 , 19 , 20 , 21. The aim of this study is to extend the clinical use of the clostridial collagenase injection as a nonsurgical therapy to fracture Dupuytren’s cords.
PATIENTS AND METHODS
Four patients, in good health, were enrolled in our study; average age was 67 years (patient 1: 72 years old, patient 2: 71 years old, patient 3: 54 years old, patient 4: 72 years old). They presented Dupuytren’s contracture of the metacarpophalangeal joint between 20° and 40°, or of the proximal interphalangeal joint between 20°and 40° in at least one digit.
Everyone of them provided written informed consent.
Exclusion criteria: breast-feeding or pregnancy, bleeding disorder, previous treatment of the primary joint within 90 days before the beginning of the study, the use of an anticoagulant within 7 days before the beginning of the study, the use of a tetracycline derivative within 14 days before the beginning of the study.
Patient 1 presented on the ring finger of the right hand flexion deformity of the metacarpophalangeal and proximal interphalangeal joints of 20° (Figures 5 and 6).
Patient 2 presented on the ring finger of the right and hand flexion deformity of the metacarpophalphalangeal joint of 20°.
Patient 3 presented for 6 months on his right hand thickening and retraction of palmar aponeurosis of 30° with nodules at the head of the ring finger. The disease was responsible of hypoeshesia and mild impairment in grip movements.
Patient 4 presented on his right hand thickening and retraction of palmar aponeurosis of 20° at the head of the ring finger.
Subjects received collagenase injection at a single dose of 10,000 U (Units).
Constitution and application were carried out according to the manufacture’s guidelines: we reconstituted collagenases in sterile diluent. Collagenases were supplied in single use glass vials containing 0.9 mg of sterile, lyophilized powder. Steps for reconstitution were carried out using aseptic technique with sterile alcohol; each vial was reconstituted with 2 mM calcium chloride in 0.9% sodium chloride USP. The reconstitution was done using different amounts of sterile diluents: 0.39 ml for cords contracting the metacarpophalangeal joint and 0.31 ml for cords contracting the proximal interphalangeal joint.
The cords to be treated were confirmed and the areas to inject were marked to prevent misapplications. The diluents were injected into the collagenase-vials and into the placebo-vial; then the vials were agitated.
Injection volumes were different depending on which joint to be treated was contracting: 0.25 ml of the solution was withdrawn using a syringe for cord contracting the metacarpophalangeal joint; 0.20 ml for cord contracting the proximal interphalangeal joint.
Before initiating treatment, the investigators identified a primary joint for treatment in each patient; secondary and tertiary joints were also identified for possible subsequent injections.
At the injection site, skin was prepared witha suitable antiseptic.
The injection was done in a point where cord was not intimately adherent to the skin, to facilitate proper administration and decrease the risk of skin tearing during the finger extension procedure.
After injection procedure, a dressing was placed over the treated hand for one day; patients were instructed to limit the motion and refrain from use of the injected hand; they were advised not to flex or extend the fingers of the injected hand, to reduce extravasation of collagenase out of the cord.
Safety assessments included monitoring vital signs and collecting blood and urine samples for immunologic measurements and routine clinical laboratory tests: patients were observed for a period of 30 minutes for potential adverse reactions or systemic hypersensitivity.
Patients were also instructed to return the following day for the passive finger extension procedure: joints were manipulated with the use of a standardized procedure in an effort to fracture the cord.
A treatment cycle comprised injection, after 24 h manipulation followed by 30-day follow-up.
Angles of extension and flexion were measured during screening, during each treatment cycle and during the follow-up visit. Also grip strength was assessed using a dynamometer during screening visit, before injection, after injection and during the follow-up visit.
RESULTS
All patients entering the trial completed the study and entered the open-label extension.
Clinical success was achieved in all of them, with a single injection of the study drug: the trial showed an initial response to injection results in reduction of joint contracture to within 0°-5° of normal in 90% of patients. Subjects were monitored for adverse response to injection, including lymphadenopathy, allergic reactions and tendon rupture. No arterial, nerve or tendon injuries were reported. There were no reports of loss of sensation, no infections and no skin grafts. Grip strength and flexion were not affected.
Complications of treatment included pain with injection and manipulation, edema and ecchymosis, injection-site swelling, itching, skin laceration. Most treatment-related adverse effects were mild or moderate in intensity and resolved without treatment over several weeks.
No patient had a recurrence of a successfully treated joint during the time of the study.
DISCUSSION
Dupuytren’s contracture can result in hand deformity and impaired function; It results from the interplay between genetic predisposition, environmental factors, local and global protein expression; it’s more common among Caucasians of Northern European and most frequently affects the ring and little fingers, followed in order of frequency by thumb, middle and index finger; occurs more often in men that in women but sex difference in prevalence diminishes with increasing age. Age, sex, ethnicity, family history and environmental factors as alcohol consumption, smoking and manual labor have been implicated in the disease. Epilepsy, diabetes mellitus, HIV infection and cancer are associated with Dupuytren’s disease 22 , 23 , 24 , 25.
In this disorder genes regulating the natural breakdown of collagen are inhibited and genes promoting structural development of collagen in the epidermis are upregulated 26 , 27.
Recent evidences demonstrate that PDGF and TGF-beta are growth factors associated with myofibrobast proliferation 28, and that metalloproteinases (in particular Gelatinase a), have effect on cell adhesion and proliferation 29 , 30 , 31;
The initial stage of the disease is characterized by the appearance of small nodular thickenings composed of myofibroblasts. Nodules over time evolve toward hypocellular bands of collagen-rich cords. The growing nodules and the arrangement of newly formatted fibers entail tissue reorganization coupled with degradation of the surrounding ECM. Patients with Dupuytren disease have also a higher rate of recurrent ectopic disease, for example Lederhose disease (plantarfibromatosis) or Peyronie disease (penile fibromatosis) 32 , 33.
Surgery is currently the most employed treatment for Dupuytren’s disease 34, but recover can be long as a result of complications, such as tendon rupture, digital nerve injury, digital artery injury and infection, hematomas, skin lesions, wound dehiscence, skin necrosis, edema, cold intolerance, pain, reflex sympathetic dystrophy, and requires postoperative hand therapy. Many patients cannot undergo surgery because of advanced age, a coexisting condition, or both. Recurrence has been reported to occur (from 26 to 80 %): it is seen more commonly in patients with early disease onset, a passive family history, rapid disease progression and distal disease. Because recurrence is high, most multiple corrective surgeries are often required 35 , 36 , 37 , 38 , 39 , 40.
Noncollagenase enzymatic fasciotomies with preparations including trypsin, hyaluronidase, and lidocaine were attempted, with limited success 41. Dupuytren’s disease may also be corrected with less-invasive procedures such as percutaneous needle aponeurotomy. Unfortunately, published data on its efficacy and safety are limited 42. A retrospective review was performed for patients with Dupuytren’s disease treated with percutaneous needle fasciotomy or collagenase: in the short term both percutaneous needle fasciotomy and collagenase have similar clinical outcomes and patient satisfaction 43. Various nonsurgical treatments have been evaluated without an effective clinical success, including radiotherapy, physical therapy, topical vitamin A and E application, anti-gout medications and interferon-gamma 44 , 45 , 46.
Steroids have shown promising results but have not been widely used as a nonsurgical treatment modality: steroids injections in Dupuytren’s nodules resulted in modification of disease progression; however, 50% patients experienced disease reactivation in 1 to 3 years 47. In a randomized, controlled study, a series of Triamcinolone acetonide injections were combined with percutaneous needle aponeurotomy in patients with Dupuytren’s contracture: it resulted in a significantly higher degree of correction; however this study provides a short-term evidence 48.
A recent evidence demonstrates that Dupuytren contracture of the proximal interphalangeal joint can be reversed by an extension torque transmitted from an external device, the Digit Widget, by skeletal pins to the middle phalanx; it gradually seems to restore the length to soft tissues palmar to the proximal interphalangeal joint’s axis of rotation 49.
Collagenase emerged as a possible agent for enzymatic fasciotomy in 1996 and offered a potential advantage over nonspecific degradative enzymes by targeting collagen. Collagenase Clostridium histolyticum is composed of two collagenases: AUX-1 and AUX-2; the two collagenases work to cleave the collagen chains and disrupt the collagen cords that cause Dupuytren’s contracture.
In vitro biochemical testing has demonstrated a 93% decrease in the tensile strenght of Dupuytren’s cords injected with collagenases 50.
In our study excessive collagen deposition in Dupuytren’s disease has been targeted by a nonoperative method using enzyme injection therapy to lyse and fracture finger cords causing metacarpophalangeal and/or proximal interphalangeal joint contractures. Collagenase-treated joints had a reduction of 50% in contracture from baseline to 30 days after injection and showed improvement in range of motion; treatment with collagenases safely and effectively restored normal finger extension in all patients. No hand therapy was required after treatment with collagenases. Little reductions of contracture were observed in placebo-treated patient: possibly performing finger flexion and extension could have improved the range of motion of placebo-treated cords.
Clearly neither surgical excision of the diseased cords nor enzymatic fasciotomy offers a definitive cure: some patients with metacarpophalangeal and proximal interphalangeal joint contractures will recur with passage of time; however the prospect of reinjection for recurrence is much less troublesome to patients than the prospect of having to undergo additional surgery 51 , 52 , 53 , 54 , 55 , 56.
A recent study analysed 2 groups of patients with Dupuytren’s contracture in the same stage of disease. Patients in the first group underwent partial fasciectomy, whereas patients in the second group were treated by injection of collagenase. Side effects like numbness, impaired blood circulation and pain were less after injection of collagenase than after partial fasciectomy. Recovery of grip strength was faster in the collagenase group than after partial fasciectomy and collagenase injection was regarded as less discomforting. Patient satisfaction was higher after collagenase injection due to subjectively perceived less negative impact and a comparable functional outcome 57.
Collagenase offers a potential advantage over nonspecific degradative enzymes by targeting collagen, and presents the potential advantages of early return of hand function and avoidance of potential surgical complications, restoring the normal finger extension in the majority of patients 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70; however, it is not without risk of complications. Some evidences report a few cases of flexon tendon ruptures and complex regional pain syndrome 71.
Compared with the well-known postoperative effects of surgery, the adverse effects of collagenase injection are of minor frequency because Clostridium histolyticum collagenase is nerve and vessel sparing, while dissolving contracture and even scar for recurrent cases. Surgery for recurrent Dupuytren contracture carries a high risk of nerve complications, infection, stiffness, and the risk of circulatory compromise and amputation 72.
Comparison between surgical approach and collagenases
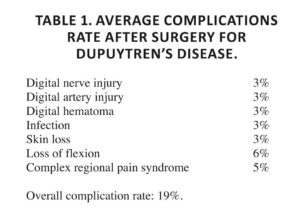
Limited fasciectomy is used to remove areas of palmar fascia affected by the disease, leaving the other areas. It represents the most frequent type of intervention. The incision must allow an exposition of all the operative field, it mustn’t determinate longitudinal scars, it must allow at the end of the intervention the extension of the fingers. It is possible to do different incisions that differ in relation to the type of intervention that the surgeon wants to do. The incision of Skoog is one of the most used. For the suture are generally used non-resorbable 4/0 or 5/0 monofilament. The incisions on the fingers are generally closed with Z multiple plastics in order to avoid the formation of longitudinal contracting scars, that could reproduce the flexion deformity. A moderately compressive bandage is placed at the center of the palm to exert the pressure at the point where it is most needed. The operated fingers are fixed on a splint in extension while the other fingers are left free. If a drainage has been introduced, the dressing should be carried out after 24 hours, otherwise after 7 days. The compression bandage should be kept in the subsequent dressings until the eighth-ninth day. The stitches are removed after about 10 days; the patient will undergo an intense period of re-education that promotes active and passive absorption of post-operative edema 73. Complications of surgical management include digital nerve injury, infection, ischemia, hematoma, stiffness, skin loss and wound-healing problems. Patients may also suffer from pain and vasomotor disorders. Pain syndroms such as Dupuytren’s “flare” and complex regional pain syndrome can be present in people with aggressive or early disease 74 , 75 (Table 1).
Use of the open palm technique has minimized the risk of these complications and is advocated by many surgeons as the procedure of choice 76.
Collagenases represent a safe and reliable treatment alternative to surgery for Dupuytren’s contracture. The use of collagenases is followed by common adverse events such as bruising, pain, swelling. Major complications rarely occur.
Flexon tendon rupture is a serious adverse event. The overall incidence of tendon rupture reported is around 0.5%. Other rare sequelas after collagenases injection are skin laceration and complex regional pain syndrome. These serious adverse events occurred in less than 1% of patients treated with collagenases 77.
This study supports a role for collagenase injection in the treatment of Dupuytren’s disease even for mild cases: collagenase is the first nonsurgical, office-based treatment option for Dupuytren’s disease with proven efficacy and safety. Patient satisfaction and physician ratings of improvement after treatment with collagenase are very high 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 showing that collagenase can be considered a cost-effective treatment 91 , 92 , 93 , 94.
CONCLUSIONS
Long term efficacy and safety of collagenase Clostridium histolyticum has been evaluated after the third year of a 5-year nontreatment follow-up study: the absence of long-term adverse events, recurrences or disease progression 3 years after the initial treatment indicates that collagease Clostridium histolyticum is an effective and safe treatment for Dupuytren’s contracture 95.
However, additional long-term studies are needed to establish the effectiveness and recurrence rate with the collagenase treatment, in comparison with palmar fasciectomy and needle aponeurectomy.
In conclusion, this study shows that a disease treatment and block of progression in patients treated with collagenases is possible. The result is probably due to the weakening of the diseased cord, obtained with collagenase followed by its fracture and by the digit extension procedure.
ACKONOWLEDGEMENTS
We thank Pfizer Italia srl for providing the product we have used. The study had on 13.06.2012 the Ethics Committee approval on protocol 1067.
This study was approved as a compassionate use study.
The following protocol for compassionate use was also sent to AIFA.
CONFLICT OF INTEREST
The Authors declare that there are no conflicts of interest.
REFERENCES
- Hughes TB, Mechrefe A, Littler JW, Akelman E. Dupuytren’s disease. Am Soc Surg Hand 2003; 3: 27-40. (back)
- Swartz WM, Lalonde DH. Dupuytren’s disease. Plast Reconstr Surg 2008; 121: 1-10. (back)
- Shaw RB, Chong AKS, Zhang A, Hentz VR, Chang J. Dupuytren’s disease: history, diagnosis and treatment. Plast Reconstr Surg 2007; 120: 44-54. (back)
- Hughes TB, Mechrefe A, Littler JW, Akelman E. Dupuytren’s disease. Am Soc Surg Hand 2003; 3: 27-40. (back)
- Swartz WM, Lalonde DH. Dupuytren’s disease. Plast Reconstr Surg 2008; 121: 1-10. (back)
- Shaw RB, Chong AKS, Zhang A, Hentz VR, Chang J. Dupuytren’s disease: history, diagnosis and treatment. Plast Reconstr Surg 2007; 120: 44-54. (back)
- Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg 2000; 25: 629-636. (back)
- Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J; CORD I Study Group. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med 2009; 361: 968-979. (back)
- Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed Collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg 2007; 32: 767-774. (back)
- Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N.Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am 2010; 35: 2027-2038. (back)
- Hueston JT. Enzymatic fasciotomy. Hand 1971; 3: 38-40. (back)
- Foucher G, Medina J, Naverro R.Percutaneous needle aponeurotomy:complications and results. J Hand Surg Br 2003; 28: 427-431. (back)
- Keilholz L, Seegenschmiedt MH, Sauer R.Radiotherpy for prevention of disease progression in early stage Dupuytren’s contracture: initial and long-term results. Int J Radiot Oncol Biol Phys 1996; 36: 891-897. (back)
- Richerds HJ. Dupuytren’s contracture treated with vitamin E. EMJ 1952; pp. 376-383. (back)
- Weinzierl G1, Flügel M, Geldmacher J. Lack of effectiveness of alternative nonsurgical treatment procedures of Dupuytren contracture. Chirurg 1993; 64: 492-494. (back)
- Howard L, Pratt D, Brunnells B.The use of compound F (hydrocortisone) in operative and nonoperative conditions of the hand. J Bone Surg 1953; 35: 994-1002. (back)
- Weinzierl G1, Flügel M, Geldmacher J. Lack of effectiveness of alternative nonsurgical treatment procedures of Dupuytren contracture. Chirurg 1993; 64: 492-494. (back)
- Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg 2000; 25: 629-636. (back)
- Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J; CORD I Study Group. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med 2009; 361: 968-979. (back)
- Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed Collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg 2007; 32: 767-774. (back)
- Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N.Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am 2010; 35: 2027-2038. (back)
- Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: non operative treatment of Dupuytren’s disease. J Hand Surg Am 2002; 27: 788-798. (back)
- Black EM, Blazar PE. Dupuytren disease: an evolving understanding of an age-old disease. J Am Acad Orthop Surg 2011; 19: 746-757. (back)
- PicardoN, Kahn W. Advances in the understanding of the aetiology of Dupuytren’s disease. Surgeon 2012; 10: 151-158. (back)
- Shih B, Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nat Rev Rheumatol 2010; 6: 715-726. (back)
- Black EM, Blazar PE. Dupuytren disease: an evolving understanding of an age-old disease. J Am Acad Orthop Surg 2011; 19: 746-757. (back)
- PicardoN, Kahn W. Advances in the understanding of the aetiology of Dupuytren’s disease. Surgeon 2012; 10: 151-158. (back)
- Badalamente MA, Sampson S, Hurst L.The role of transforming growth factor b in Dupuytren’s disease. J Hand Surg 1996; 21: 210-215. (back)
- Wilkinson J, Davidson R, Swingler T. MMP-14 and MMP-2 are key metalloproteases in Dupuytren’s disease fibroblast mediated contraction. Biochim Biophys Acta 2012; 1822: 897-905. (back)
- Johnston P, Chojnowski AJ, Davidson RK, Riley GP, Donell ST, Clark IM. A complete expression profile of matrix-degrading metalloproteinases in Dupuytren’s disease. J Hand Surg 2007; 32: 343-351. (back)
- Augoff K, Ratajczak K, Gosk J, Tabola R, Rutowski R. Gelatinase A activity in Dupuytren’s disease. J Hand Surg Am 2006; 31: 1635-1639. (back)
- Black EM, Blazar PE. Dupuytren disease: an evolving understanding of an age-old disease. J Am Acad Orthop Surg 2011; 19: 746-757. (back)
- PicardoN, Kahn W. Advances in the understanding of the aetiology of Dupuytren’s disease. Surgeon 2012; 10: 151-158. (back)
- Howard MD, Beredjiklian PK. 50 years ago in CORR: Dupuytren’s contracture: a guide for management.Clin Orthop Relat Res 2011; 469: 309-311. (back)
- Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg 2000; 25: 629-636. (back)
- Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J; CORD I Study Group. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med 2009; 361: 968-979. (back)
- Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed Collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg 2007; 32: 767-774. (back)
- Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N.Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am 2010; 35: 2027-2038. (back)
- Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: non operative treatment of Dupuytren’s disease. J Hand Surg Am 2002; 27: 788-798. (back)
- Shih B, Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nat Rev Rheumatol 2010; 6: 715-726. (back)
- Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed Collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg 2007; 32: 767-774. (back)
- Foucher G, Medina J, Naverro R.Percutaneous needle aponeurotomy:complications and results. J Hand Surg Br 2003; 28: 427-431. (back)
- Nydick JA, Olliff BW, Garcia MJ, Hess AV, Stone JD.A comparison of percutaneous needle fasciotomy and collagenase injection for Dupuytren disease. J Hand Surg Am 2013; 38: 2377-2380. (back)
- Keilholz L, Seegenschmiedt MH, Sauer R.Radiotherpy for prevention of disease progression in early stage Dupuytren’s contracture: initial and long-term results. Int J Radiot Oncol Biol Phys 1996; 36: 891-897. (back)
- Richerds HJ. Dupuytren’s contracture treated with vitamin E. EMJ 1952; pp. 376-383. (back)
- Weinzierl G1, Flügel M, Geldmacher J. Lack of effectiveness of alternative nonsurgical treatment procedures of Dupuytren contracture. Chirurg 1993; 64: 492-494. (back)
- Howard L, Pratt D, Brunnells B.The use of compound F (hydrocortisone) in operative and nonoperative conditions of the hand. J Bone Surg 1953; 35: 994-1002. (back)
- McMillan C, Binhammer P. Steroid injection and needle aponeurotomy for Dupuytren contracture: a randomized, controlled study. J Hand Surg 2012; 37: 1307-1312. (back)
- Agee JM, Goss BC.The use of skeletal Extension Torque in reversing Dupuytren Contractures of the proximal interphalangeal joint. J Hand Surg 2012; 37: 1467-1474. (back)
- Starkweather KD, Lattuga S, Hurst LC, Badalamente MA, Guilak F, Sampson SP, Dowd A, Wisch D. Collagenase in the treatment of Dupuytren’s disease: an in vitro study. J Hand Surg 1996; 21: 490-495. (back)
- Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg 2000; 25: 629-636. (back)
- Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J; CORD I Study Group. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med 2009; 361: 968-979. (back)
- Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed Collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg 2007; 32: 767-774. (back)
- Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N.Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am 2010; 35: 2027-2038. (back)
- Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: non operative treatment of Dupuytren’s disease. J Hand Surg Am 2002; 27: 788-798. (back)
- Shih B, Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nat Rev Rheumatol 2010; 6: 715-726. (back)
- Vollbach FH, Walle L, Fansa H. Dupuytren’s disease-patient satisfaction and functional results one year after partial fasciectomy and injection of collagenase. Handchir Mikrochir Plast Chir 2013; 45: 258-264. (back)
- Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg 2000; 25: 629-636. (back)
- Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J; CORD I Study Group. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med 2009; 361: 968-979. (back)
- Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed Collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg 2007; 32: 767-774. (back)
- Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N.Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am 2010; 35: 2027-2038. (back)
- Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: non operative treatment of Dupuytren’s disease. J Hand Surg Am 2002; 27: 788-798. (back)
- Watt AJ, Curtin CM, Hentz VR.Collagenase injection as nonsurgical treatment of Dupuytren’s disease: 8-year follow-up. J Hand Surg 2010; 35: 534-539. (back)
- Foissac R, Camuzard O, Dumas P, Dumontier C, Chignon-Sicard B. Treatment of Dupuytren’s contracture by collagenase injection. Chir Main 2013; 32: 199-205. (back)
- Martín-Ferrero MÁ, Simón-Pérez C, Rodríguez-Mateos JI, García-Medrano B, Hernández-Ramajo R, Brotat-García M. Treatment of Dupuytren’s disease using collagenase from Clostridium histolyticum Rev Esp Cir Ortop Traumatol 2013; 57: 398-402. (back)
- Witthaut J, Jones G, Skrepnik N, Kushner H, Houston A, Lindau TR. Efficacy and safety of collagenase Clostridium histolyticum injection for Dupuytren contracture; short-term results from 2 open-label studies. J Hand Surg Am 2013; 38: 2-11. (back)
- Bendon CL, Giele HP. Collagenase for Dupuytren’s disease of the thumb. J Bone Joint Surg Br 2012; 94: 1390-1392. (back)
- Azzopardi E, Boyce DE. Clostridium histolyticum collagenase in the treatment of Dupuytren’s contracture. Br J Hosp Med (Lond) 2012; 73: 432-436. (back)
- [No authors listed]. Xiapex for Dupuytren’s contracture. Drug Ther Bull 2011; 49: 138-141. (back)
- Spanholtz TA, Holzbach T, Wallmichrath J, Pototschnig A, Deglmann C, Frick A, Giunta RE. Treatment of Dupuytren’s contracture by means of injectable collagenase: first clinical experiences. Handchir Mikrochir Plast Chir 2011; 43: 275-280. (back)
- Zhang AY, Curtin CM, Hentz VR. Flexor tendon rupture after collagenase injection for dupuytren contracture: case report. J Hand Surg 2011; 36: 1323-1325. (back)
- Denkler K. Collagenase for recurrent dupuytren contracture with skin grafts. J Hand Surg Am 2013; 38: 1264. (back)
- Santarelli F, Scuderi N. Chirurgia Plastica Ricostruttiva ed Estetica (e chirurgia plastica). Edizioni Luigi Pozzi 2007; Cap II: 133-135. (back)
- Shaw RB, Chong AKS, Zhang A, Hentz VR, Chang J. Dupuytren’s disease: history, diagnosis and treatment. Plast Reconstr Surg 2007; 120: 44-54. (back)
- Denkler K. Collagenase for recurrent dupuytren contracture with skin grafts. J Hand Surg Am 2013; 38: 1264. (back)
- Swartz WM, Lalonde DH. Dupuytren’s disease. Plast Reconstr Surg 2008; 121: 1-10. (back)
- Zhang AY, Curtin CM, Hentz VR. Flexor tendon rupture after collagenase injection for dupuytren contracture: case report. J Hand Surg 2011; 36: 1323-1325. (back)
- Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg 2000; 25: 629-636. (back)
- Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J; CORD I Study Group. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med 2009; 361: 968-979. (back)
- Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed Collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg 2007; 32: 767-774. (back)
- Gilpin D, Coleman S, Hall S, Houston A, Karrasch J, Jones N.Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am 2010; 35: 2027-2038. (back)
- Badalamente MA, Hurst LC, Hentz VR. Collagen as a clinical target: non operative treatment of Dupuytren’s disease. J Hand Surg Am 2002; 27: 788-798. (back)
- Watt AJ, Curtin CM, Hentz VR.Collagenase injection as nonsurgical treatment of Dupuytren’s disease: 8-year follow-up. J Hand Surg 2010; 35: 534-539. (back)
- Foissac R, Camuzard O, Dumas P, Dumontier C, Chignon-Sicard B. Treatment of Dupuytren’s contracture by collagenase injection. Chir Main 2013; 32: 199-205. (back)
- Martín-Ferrero MÁ, Simón-Pérez C, Rodríguez-Mateos JI, García-Medrano B, Hernández-Ramajo R, Brotat-García M. Treatment of Dupuytren’s disease using collagenase from Clostridium histolyticum Rev Esp Cir Ortop Traumatol 2013; 57: 398-402. (back)
- Witthaut J, Jones G, Skrepnik N, Kushner H, Houston A, Lindau TR. Efficacy and safety of collagenase Clostridium histolyticum injection for Dupuytren contracture; short-term results from 2 open-label studies. J Hand Surg Am 2013; 38: 2-11. (back)
- Bendon CL, Giele HP. Collagenase for Dupuytren’s disease of the thumb. J Bone Joint Surg Br 2012; 94: 1390-1392. (back)
- Azzopardi E, Boyce DE. Clostridium histolyticum collagenase in the treatment of Dupuytren’s contracture. Br J Hosp Med (Lond) 2012; 73: 432-436. (back)
- [No authors listed]. Xiapex for Dupuytren’s contracture. Drug Ther Bull 2011; 49: 138-141. (back)
- Spanholtz TA, Holzbach T, Wallmichrath J, Pototschnig A, Deglmann C, Frick A, Giunta RE. Treatment of Dupuytren’s contracture by means of injectable collagenase: first clinical experiences. Handchir Mikrochir Plast Chir 2011; 43: 275-280. (back)
- Chen NC, Shauver MJ, Chung KC. Cost-effectiveness of open partial fasciectomy, needle aponeurotomy, and collagenase injection for Dupuytren contracture. J Hand Surg Am 2011; 36: 1827-1834. (back)
- Balzer H, Binhammer PA. Cost-effectiveness in the management of Dupuytren’s contracture. A Canadian cost-utility analysis of current and future management strategies. Bone Joint J 2013; 95: 1094-1100. (back)
- De Salas-Cansado M, Cuadros M, Del Cerro M, Arandes JM. Budget impact analysis in Spanish patients with Dupuytren’s contracture: fasciotomy vs. collagenase Clostridium histolyticum. Chir Main 2013; 32: 68-73. (back)
- Wiwanitkit V. Cost of open partial fasciectomy, needle aponeurotomy, and collagenase injection for dupuytren contracture. J Hand Surg Am 2012; 37: 394. (back)
- Peimer CA, Blazar P, Coleman S, Kaplan FT, Smith T, Tursi JP, Cohen B, Kaufman GJ, Lindau T. Dupuytren contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS Study): 3-year data. J Hand Surg Am 2013; 38: 12-22. (back)
To cite this article
Injectable Clostridium Histolyticum Collagenases for Mild Dupuytren’s Contracture: Preliminary Data
CellR4 2014; 2 (4): e1090
Publication History
Published online: 26 Jul 2014

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.